Figure 2.
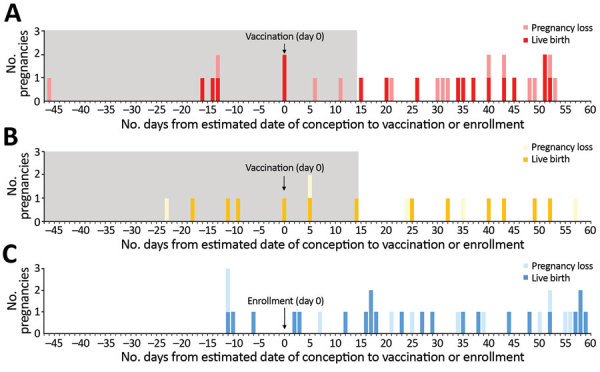
Number of pregnancies by estimated date of conception relative to vaccination or enrollment among 81 participants in the Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE). A) Immediate vaccination group (n = 31). B) Deferred crossover vaccination group (n = 17). C) Unvaccinated group (n = 33). Because pregnancy outcome for 3 of the 84 women was unknown, these 3 women are not included in the figure. Outcomes include live birth (term and preterm) and pregnancy loss (early and late loss). Gray shaded area denotes the high viremia risk period (i.e., women who were pregnant when vaccinated or became pregnant 0–14 days after vaccination).
