Fig. 4. Pharmacokinetic Study of CTB-ACE2/Ang-(1–7) following oral dose administrations.
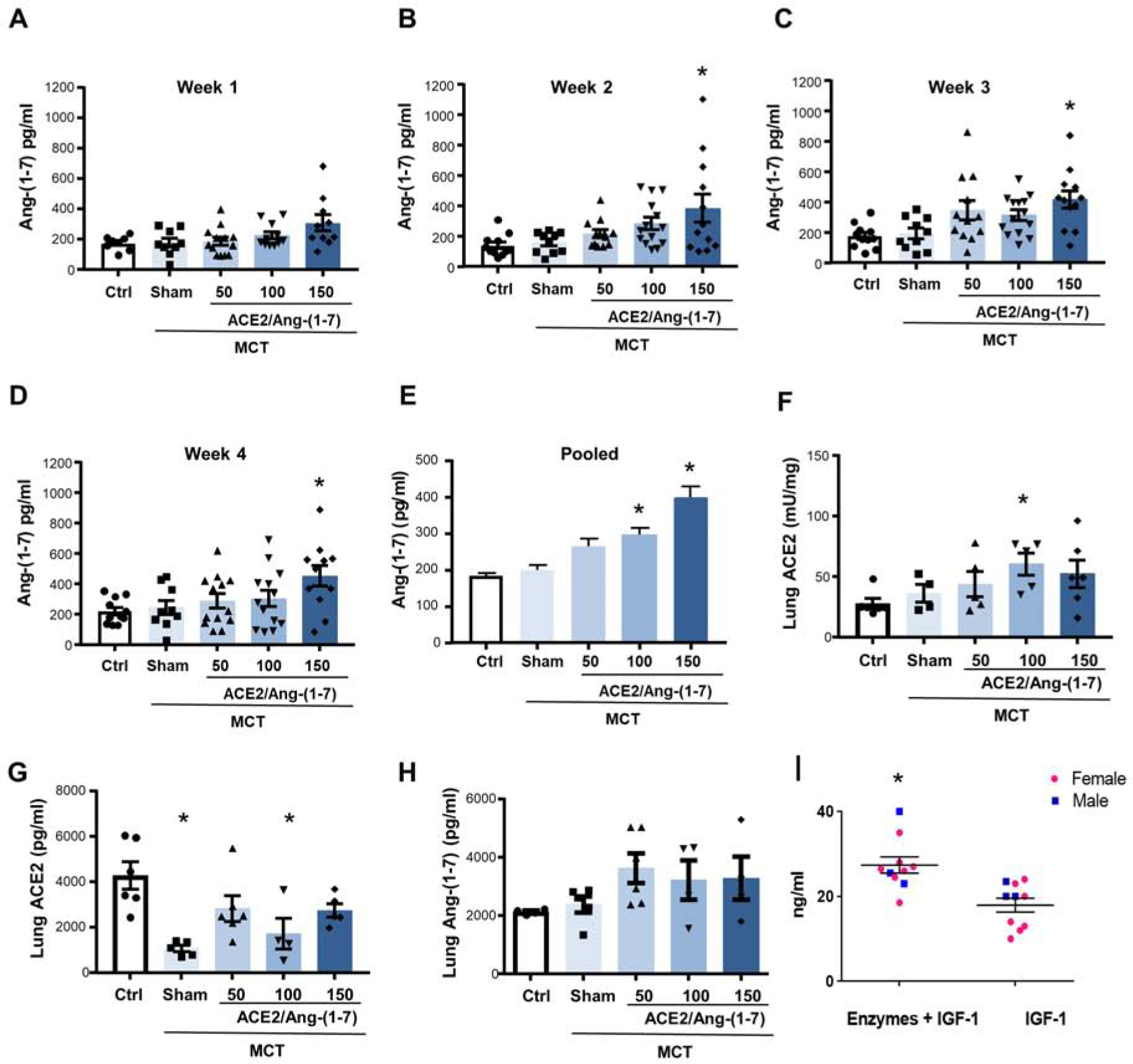
Increases of Ang-(1–7) plasma levels in rats with monocrotaline-induced pulmonary hypertension by combinational feeding with bioencapsulated CTB-ACE2 and CTB-Ang-(1–7). CTB-ACE2 (50, 100 or 150 mg of plant material; containing 0.4, 0.8 and 1.2 mg of ACE2 protein) and CTB-Ang-(1–7) (50, 100 or 150 mg of plant material; containing 0.25, 0.5 and 0.7 mg of Ang-(1–7) protein) were gavaged daily. Animals in sham group were gavaged with PBS only. Levels were measured once a week 5h after gavage. (A-D) Absolute Ang-(1–7) plasma levels for each week (1 to 4). Each data point represents one animal. (E) Pooled Ang-(1–7) plasma levels (combined data of week 1 to 4) are shown. (F-H) Lung ACE2 activity and quantity as well as lung Ang-(1–7) levels. Error bars or values represent ± SEM. #p < 0.05 vs. MCT groups by one-way ANOVA and post-hoc Newman-Keuls test. Ctrl = untreated control; MCT = monocrotaline. (I) C57BL/6J mice were gavaged with the enzymes mannanase, pectinate lyase, endoglucanase and exoglucanase (5 mg plant powder/enzyme) and one hour later gavaged with PTD-Pro-IGF-1 (5ug IGF-1/20mg plant powder). Controls were gavaged with PBS followed by PTD-Pro IGF-1. *p=0.009.
