Figure 3.
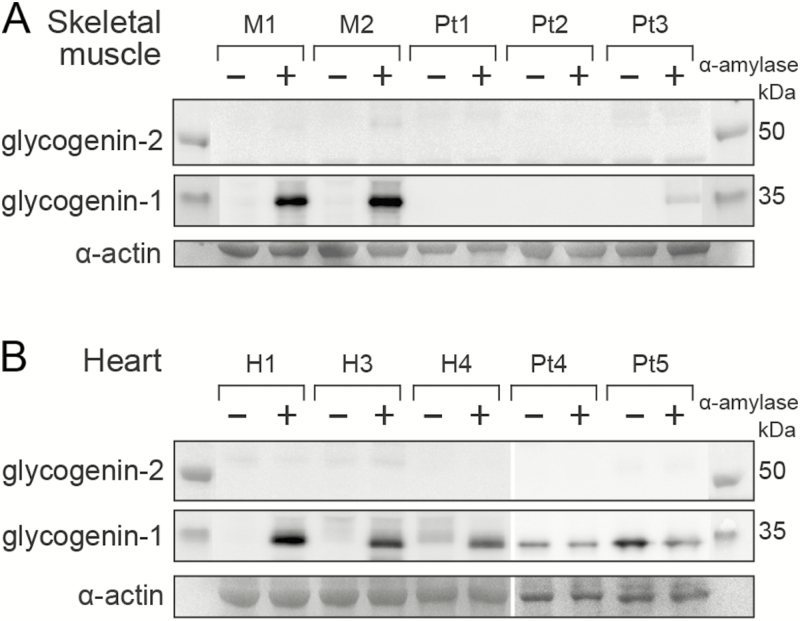
Western blot analysis of glycogenin-1 and glycogenin-2 in patients with biallelic GYG1 mutations. A, In skeletal muscle, glycogenin-1 was detected only in controls (M1-M2) after α-amylase treatment (+). However, a weak band of normal size was seen after α-amylase treatment in Pt3, indicating a small amount of functional glycogenin-1. Glycogenin-2 was not detected in controls or in patients with GYG1 mutations (Pt1-Pt3) irrespective of whether there was α-amylase treatment. B, In the heart, glycogenin-1 was detectable after α-amylase treatment (+) in controls (H1, H3, and H4) as well as patients (Pt4 and Pt5). Glycogenin-1 is nonfunctional in the heart in patients with biallelic GYG1 mutations because it was identified with equally strong bands both with (+) and without (–) α-amylase treatment. It was smaller (≈1 kDa) than the glycogenin-1 identified in 1 of the control hearts without α-amylase treatment (see H4 [–]), indicating that the glycogenin-1 in the patients was not capable of autoglucosylation and therefore not functional. Glycogenin-2 was not detected in controls or patients. The loading controls were α-actin from gels stained with Coomassie blue (SimplyBlue SafeStain,Thermo Fisher Scientific). Pt, patient.