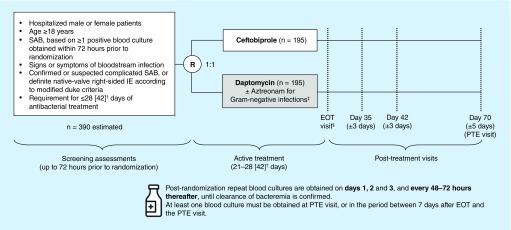
Figure 1. . Study design.
†Patients in Part 1 (N = 80) will receive active treatment for 21–28 days. For Part 2, maximum duration of therapy may be extended to 42 days, pending the outcome of the interim safety analysis and implementation of a protocol amendment.
‡Patients in the ceftobiprole group with Gram-negative infections receive placebo to maintain blinding.
§EOT visit to be conducted within 72 h of last study-drug administration.
EOT: End of treatment; IE: Infective endocarditis; PTE: Post-treatment evaluation; R: Randomization; SAB: Staphylococcus aureus bacteremia.