Extended Data Fig. 4. The LytH-ActH complex has peptidoglycan hydrolase activity.
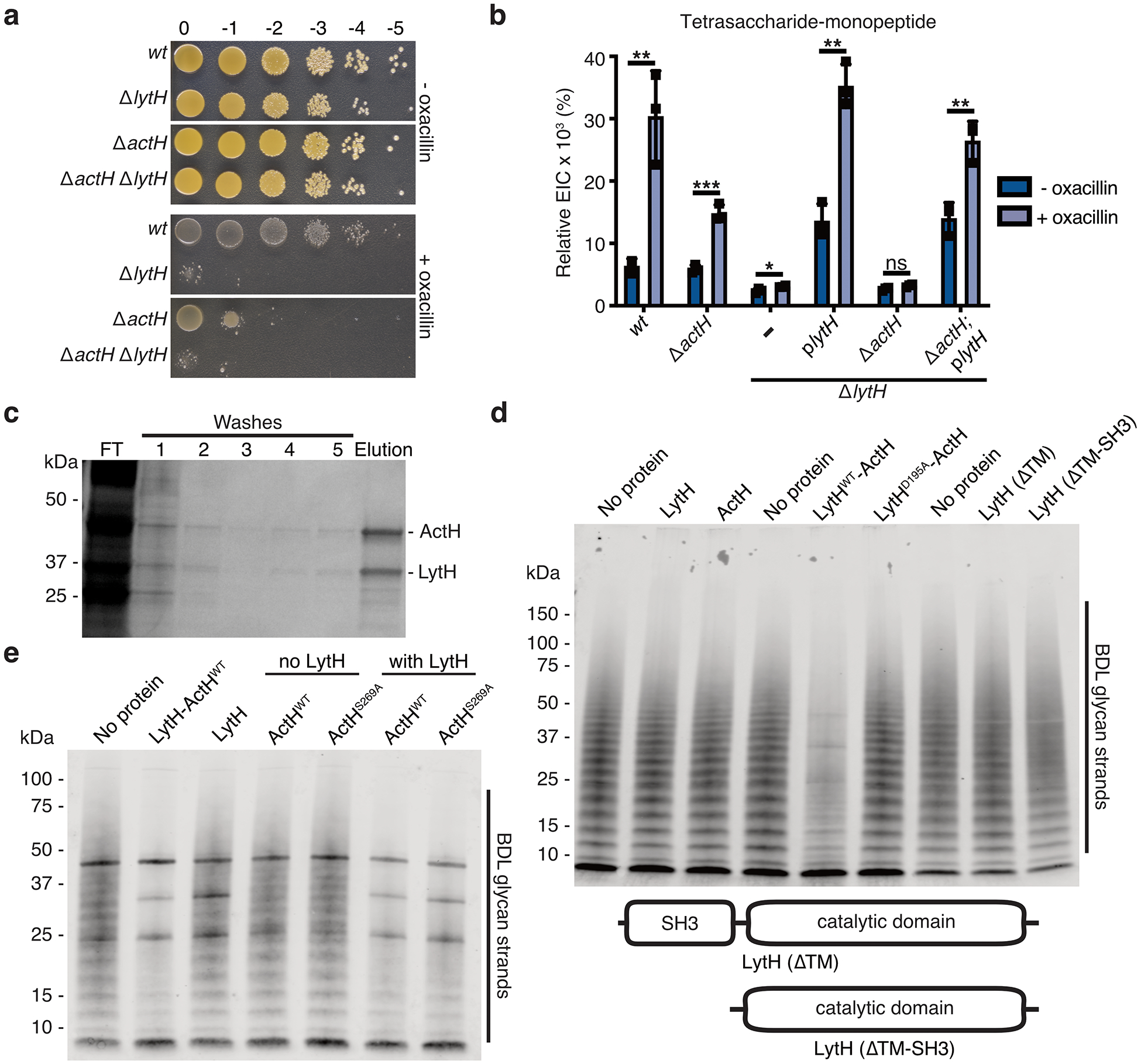
a, HG003 strains were spotted on TSA ± 0.125 μg/mL oxacillin. b, The relative ion count (extracted ion count/total ion count) for the tetrasaccharide-monopeptide was calculated. Data represent the mean ± SD. P-values were determined using unpaired, two-tailed t-tests (*P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant). From left to right: P = 0.0048, 0.0005, 0.0387, 0.0010, 0.0580, and 0.0056. c, Full-length LytH and ActH were co-expressed in E. coli and purified from detergent-solubilized membranes as a stable complex. SDS-PAGE analysis of purification fractions: (FT) flowthrough. d, Western blot analysis of LytH reactions with glycan strand substrate, showing a distinct difference for the WT complex. Glycan strands were made in vitro from native S. aureus Lipid II; the strands were labeled with BDL for visualization. e, Western blot analysis of LytH reactions with glycan strand substrate, showing the catalytic serine of ActH is dispensable for activating LytH. The bold bands appearing in all lanes in the blot correspond to purified proteins that were added to the reactions and non-specifically labeled. Data are representative of three (b–c) and two (a, d–e) independent experiments.
