Table 3.
Forest Plot of the Cumulative Weighted Mean Effects for Blood Pressure Based on Start Date of Data Collection.
| Cumulative Statistics | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study Citation | Outcome | N | Date | Point | SE | Variance | 95% CI | Cumulative d+ (95% CI) | |
| Lower | Upper | ||||||||
| Schneider et al. (1995) | SBP | 74 | 1989 | 0.9716 | 0.2501 | 0.0626 | 0.4814 | 1.4618 | 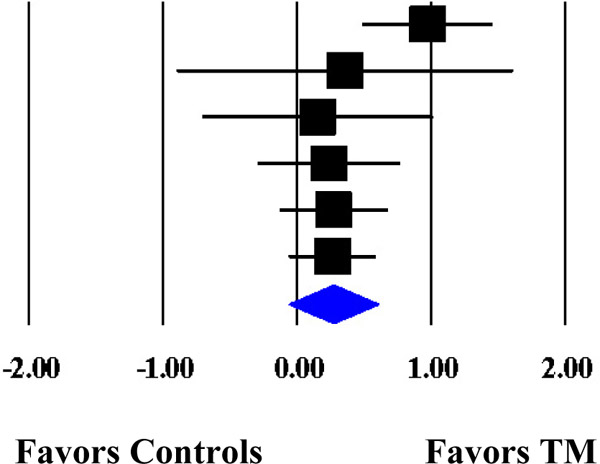 |
| Kondwani et al. (2005) | SBP | 34 | 1992 | 0.3556 | 0.6414 | 0.4114 | −0.9015 | 1.6126 | |
| Calderon (2000) | SBP | 71 | 1995 | 0.1503 | 0.4417 | 0.1951 | −0.7155 | 1.0160 | |
| Schneider et al. (2012) | SBP | 183 | 1998 | 0.2349 | 0.2733 | 0.0747 | −0.3008 | 0.7705 | |
| Paul-Labrador et al. (2006) | SBP | 84 | 1999 | 0.2715 | 0.2075 | 0.0430 | −0.1351 | 0.6781 | |
| Schneider et al. (2005) | SBP | 98 | 1999† | 0.2636 | 0.1668 | 0.0278 | −0.0633 | 0.5905 | |
| MM | 0.2636 | 0.1668 | 0.0278 | −0.0633 | 0.5905 | ||||
| ML | 0.2673 | 0.1547 | 0.0239 | −0.0358 | 0.5704 | ||||
| Schneider et al. (1995) | DBP | 74 | 1989 | 0.8427 | 0.2466 | 0.0608 | 0.3594 | 1.3260 | 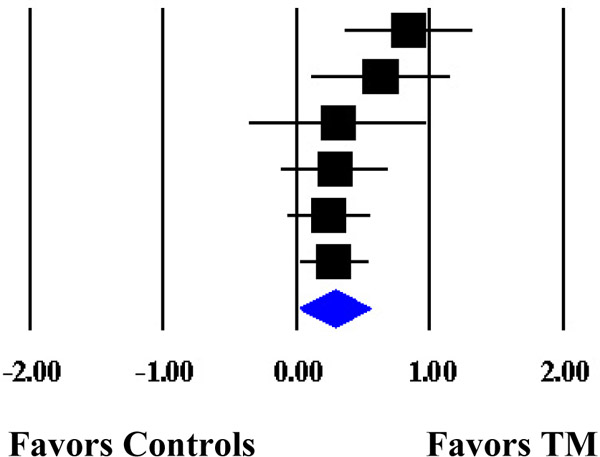 |
| Kondwani et al. (2005) | DBP | 34 | 1992 | 0.6326 | 0.2653 | 0.0704 | 0.1127 | 1.1525 | |
| Calderon (2000) | DBP | 71 | 1995 | 0.3134 | 0.3419 | 0.1169 | −0.3567 | 0.9835 | |
| Schneider et al. (2012) | DBP | 183 | 1998 | 0.2904 | 0.2071 | 0.0429 | −0.1154 | 0.6962 | |
| Paul-Labrador et al. (2006) | DBP | 84 | 1999 | 0.2429 | 0.1622 | 0.0263 | −0.0751 | 0.5609 | |
| Schneider et al. (2005) | DBP | 98 | 1999† | 0.2837 | 0.1361 | 0.0185 | 0.0170 | 0.5504 | |
| MM | 0.2837 | 0.1361 | 0.0185 | 0.0170 | 0.5504 | ||||
| ML | 0.2848 | 0.1571 | 0.0247 | −0.0231 | 0.5926 | ||||
Note. The cumulative weighted mean effect sizes are calculated using random-effects models with methods of moments (MM) or full information maximum likelihood (ML) to estimate the between-study variance. Weighted mean effect sizes (d+) are positive for differences that favor the transcendental meditation (TM) group relative to controls. Date refers to the year data collection commenced. d+, weighted mean effect size; SE, standard error; CI, confidence interval; SBP, systolic blood pressure; DBP, diastolic blood pressure.
The start and end dates for the study trial were not provided and therefore we used the start date of the study funding period.
