Abstract
Background:
The platelet to lymphocyte ratio (PLR) has recently emerged as a potential inflammatory biomarker and has been shown to be significantly associated with atherosclerotic coronary artery disease (CAD). Therefore, we aimed to explore the association of PLR with in-hospital major adverse cardiovascular events (MACEs) and the severity of CAD assessed by the Gensini score (GS) in patients with acute myocardial infarction (AMI) undergoing coronary angiography.
Methods:
A total of 502 patients with AMI consecutively treated at the Affiliated Hospital of Qingdao University (Qingdao, China) and underwent coronary angiography from August 2017 to December 2018 were recruited in this study. The demographic, clinical, angiographic characteristics, and laboratory parameters were collected. According to the presence of in-hospital MACEs, the included patients were divided into the MACE group (n = 81) and the non-MACE group (n = 421). Further, according to tertiles of the GS, the patients were classified into three groups: the low GS group (GS ≤ 32 points, n = 173), medium GS group (32 points < GS ≤ 60 points, n = 169), and high GS group (60 points < GS ≤ 180 points, n = 160). The main statistical methods included Chi-squared test, non-parametric Mann-Whitney U test, Kruskal-Wallis H test, logistic regression, and receiver operating characteristic curves.
Results:
The PLR in the MACE group was significantly higher than that in the non-MACE group (179.43 [132.84, 239.74] vs. 116.11 [87.98, 145.45], Z = –8.109, P < 0.001). Further, there were significant differences in PLR among the tertiles of GS (110.05 [84.57, 139.06] vs. 119.78 [98.44, 157.98] vs. 140.00 [102.27, 191.83], H = 19.524, P < 0.001). PLR was demonstrated to be an independent risk factor of in-hospital MACEs (odds ratio [OR]: 1.012, 95% confidential interval [CI]: 1.006–1.018, P < 0.001) and severe CAD assessed by the GS (OR: 1.004, 95% CI: 1.002–1.009, P = 0.042). The cutoff value of PLR for predicting the development of in-hospital MACEs was 151.28 with a sensitivity of 66.7% and a specificity of 78.1% (area under the curve [AUC]: 0.786, 95% CI: 0.730–0.842, P < 0.001), and a PLR of 139.31 was also identified to be an effective cutoff point for detecting a high GS (>60 points) with a sensitivity of 49.4% and a specificity of 69.6% (AUC: 0.611, 95% CI: 0.556–0.666, P < 0.001).
Conclusions:
PLR as a novel inflammatory marker is significantly and independently associated with the occurrence of in-hospital MACEs and the severity of CAD assessed by the GS in patients with AMI. As an easily available and inexpensive inflammatory indicator, PLR could be widely used as an efficient inflammatory biomarker for identifying high-risk patients and for individualizing targeted therapy to improve the prognosis of AMI.
Keywords: Platelet to lymphocyte ratio, Major cardiovascular adverse event, Gensini score, Myocardial infarction
Introduction
Acute myocardial infarction (AMI) is mainly caused by the rupture of a coronary atherosclerotic plaque or thrombosis, which leads to acute complete occlusion of the vascular cavity and necrosis of myocardial cells. As an important type of coronary artery disease (CAD), AMI is a leading cause of morbidity and mortality worldwide. Despite the improvements in reperfusion strategies, the outcome of patients with AMI remains unsatisfactory. Thus, early risk stratification and timely intervention measures are of great significance in improving the prognosis of patients with AMI.
Present studies have shown that inflammatory processes play an important role in atherosclerosis. As a novel inflammatory marker, the platelet to lymphocyte ratio (PLR) has been reported to be strongly associated with CAD. PLR has been showed distinct predictive value on mortality or major adverse cardiovascular events (MACEs)[1,2] and the severity of CAD was likewise demonstrated to be related to PLR.[3,4]The aim of this study was to investigate the potential association between PLR and in-hospital MACEs as well as CAD severity assessed by the Gensini score (GS) in patients with AMI.
Methods
Ethical approval
The study protocol complied with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (No. QYFY WZLL 25627). Because this was a retrospective study and the data analysis was performed anonymously, this study was exempt from informed consent from patients.
Study population
A total of 597 consecutive patients diagnosed with AMI and admitted to the Affiliated Hospital of Qingdao University from August 2017 to December 2018 were successively recruited in this retrospective study. All patients met the diagnostic criteria for AMI, which were based on the fourth global definition of myocardial infarction.[5] All patients underwent coronary angiography with or without percutaneous coronary intervention (PCI) and were simultaneously treated with effective expectation.
Patients who were already using fibrinolytic agents before being referred for primary PCI; those with a history of previous AMI or coronary revascularization (either coronary artery bypass graft surgery or PCI); those complicated with other cardiac diseases (including severe congenital heart disease, valvular heart disease, and cardiomyopathy); those with any hematological disease including anemia, any systemic inflammatory disease or autoimmune disorder, malignancy, severe renal and/or hepatic insufficiency, or recent infection; those with use of non-steroidal anti-inflammatory drugs in the previous week or steroids (including steroid creams) in the previous 6 months were excluded. After the elimination according to the exclusion criteria, the remaining 502 patients were finally recruited in the present study.
Clinical information
The patients’ demographic data (age, sex, body mass index [BMI]), information on risk factors for CAD (smoking, hypertension, and diabetes mellitus [DM]) and information on previous medications were collected after admission.
BMI (weight [kg]/height squared [m2]) was calculated for all patients. Those who smoked one or more cigarettes per day were categorized as smokers. Hypertension was diagnosed based on repeated blood pressure measurements of systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg (at least two times in different environments) or use of antihypertensive drugs. Patients using oral anti-diabetic agents or insulin injections or with a fasting serum glucose level of ≥126 mg/dL (7.0 mmol/L) were considered to have DM. MACE refers to the occurrence of acute cardiac failure, severe arrhythmias (ventricular tachycardia/ventricular fibrillation and severe conduction block), non-fatal myocardial infarction, and death.
Fasting peripheral venous blood was collected in the morning of the second day after admission, and all data were obtained from the same blood sample. The neutrophil to lymphocyte ratio (NLR) was calculated by dividing the neutrophil count by lymphocyte count and the PLR was computed using the absolute platelet count divided by the absolute lymphocyte count.
Angiographic examinations
Selective coronary angiography was performed in all included patients using the standard Judkin's technique. The severity of CAD was assessed based on the GS.[6] The coronary angiograms were analyzed and the GS was determined by two interventional cardiologists who were blinded to the clinical and laboratory data of the patients. In the case of a disagreement, a third interventional cardiologist who was unaware of the laboratory results and the nature of the study evaluated the coronary angiograms and determined the GS. The number of diseased vessels was also recorded, and significant left main coronary artery lesion was considered the equivalent of three-vessel CAD.
Statistical analysis
Statistical analyses were performed using SPSS software, version 25.0 (IBM, New York, NY, USA). The normality of the distribution of continuous variables was tested using the Kolmogorov-Smirnov test and Q-Q plots. Non-normally distributed continuous variables are expressed as medians with interquartile range (quartiles 1–3). Categorical variables are presented as counts and percentages. Mann-Whitney U test was used to compare non-normally distributed continuous variables between the MACE and non-MACE groups, whereas comparisons of non-normally distributed continuous variables among the GS tertiles were performed with Kruskal-Wallis H test. Categorical variables were compared using the Chi-squared test. Univariate and multivariate regression analyses were performed to analyze the risk factors of in-hospital MACEs and severe CAD assessed by the GS. Receiver-operating characteristic (ROC) curve analysis was also performed to determine the optimal cutoff values of PLR for predicting in-hospital MACEs and high GS. All P-values were two-tailed, and a probability of <0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 502 consecutive patients with AMI referred for coronary angiography from August 2017 to December 2018 at the Affiliated Hospital of Qingdao University were finally included in the analysis.
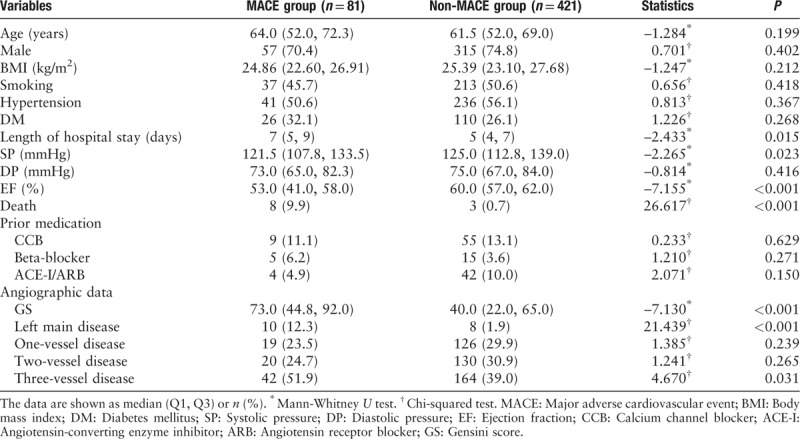
The baseline demographic, clinical, and angiographic characteristics of the MACE and non-MACE groups are summarized in Table 1. As shown in Table 1, the two groups were similar in terms of age, sex, BMI, smoking habit, hypertension, DM, and previous medication (including calcium channel blocker, angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker, and beta-blocker) (all P > 0.05). The percentages of death, left main disease, and three-vessel disease were significantly higher in the MACE group than those in the non-MACE group (P < 0.001, P < 0.001, and P = 0.031, respectively). The length of hospital stay (7 [5, 9] days vs. 5 [4, 7] days, Z = –2.433, P = 0.015), admission systolic pressure (121.5 [107.8, 133.5] mmHg vs.125.0 [112.8, 139.0] mmHg, Z = –2.265, P = 0.023), ejection fraction (53.0 [41.0, 58.0] % vs. 60.0 [57.0, 62.0] %, Z = –7.155, P < 0.001), and GS (73.0 [44.8, 92.0] vs. 40.0 [22.0, 65.0], Z = –7.130, P < 0.001) were significantly different between the two study groups.
Table 1.
Baseline demographic, clinical, and angiographic characteristics of the study population according to in-hospital major adverse cardiovascular events.
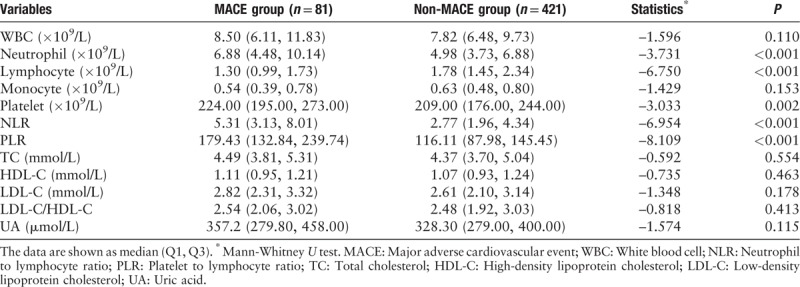
The laboratory parameters of the two patient groups are summarized in Table 2. No significant differences were observed in white blood cell (WBC) count, monocyte count, total cholesterol level, high-density lipoprotein cholesterol (HDL-C) level, low-density lipoprotein cholesterol (LDL-C) level, LDL-C/HDL-C ratio, and uric acid level between the two groups (all P > 0.05). The NLR and PLR values were significantly higher in the MACE group than in the non-MACE group (5.31 [3.13, 8.01] vs. 2.77 [1.96, 4.34], Z = –6.954, P < 0.001 and 179.43 [132.84, 239.74] vs. 116.11 [87.98, 145.45], Z = –8.109, P < 0.001, respectively), as expected. In addition, the neutrophil count (6.88 [4.48, 10.14] × 109/L vs. 4.98 [3.73, 6.88] × 109/L, Z = –3.731, P < 0.001) and platelet count (224.0 [195.0, 273.0] × 109/L vs. 209.0 [176.0, 244.0] × 109/L, Z = –3.033, P = 0.002) were significantly higher in the MACE group than in the non-MACE group, and the lymphocyte count (1.30 [0.99, 1.73] × 109/L vs. 1.78 [1.45, 2.34] × 109/L, Z = –6.750, P < 0.001) was significantly lower in the MACE group than in the non-MACE group.
Table 2.
Laboratory parameters of the study population according to in-hospital major adverse cardiovascular events.
According to the GS tertiles, the included patients were classified into three groups: the low GS group (GS ≤ 32 points, n = 173), medium GS group (32 points < GS ≤60 points, n = 169), and high GS group (60 points < GS ≤ 180 points, n = 160), the low GS group and medium GS group were defined as non-high GS group. The baseline demographic, clinical, and laboratory data of the study population are shown in Table 3. Age, sex, BMI, smoking habit, hypertension, length of hospital stay, systolic pressure, diastolic pressure, and laboratory parameters (including WBC count, neutrophil count, monocyte count, and uric acid level) were similar among the groups classified according to the GS (all P > 0.05). There were significant differences in the prevalence of DM and the ejection fraction among the three groups (P = 0.001 and P < 0.001, respectively).
Table 3.
Comparison of demographic, clinical, and laboratory characteristics among the low Gensini score tertile (Group 1), medium Gensini score tertile (Group 2), and high Gensini score tertile (Group 3).
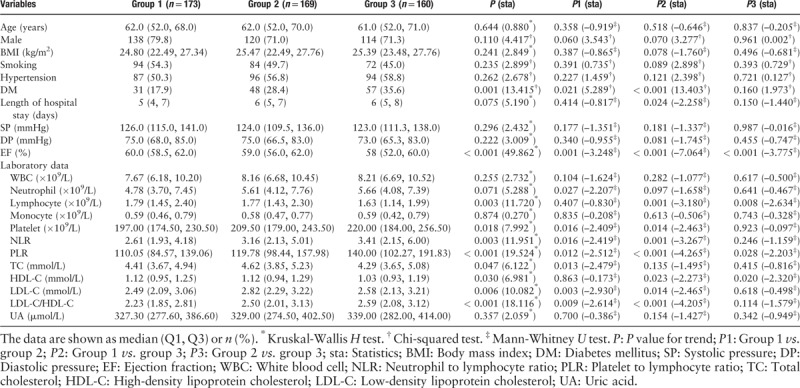
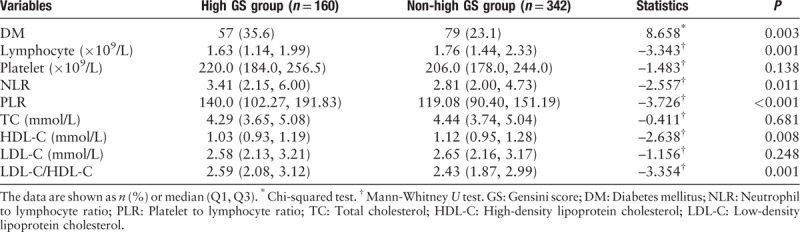
As demonstrated in Table 3, among the three groups based on the GS, there were also significant differences in NLR, PLR, lymphocyte count, platelet count, total cholesterol level, HDL-C level, LDL-C level, and LDL-C/HDL-C ratio (P = 0.003, P < 0.001, P = 0.003, P = 0.018, P = 0.047, P = 0.030, P = 0.006, and P < 0.001, respectively). More importantly, the NLR, PLR, HDL-C level, and LDL-C/HDL-C ratio were significantly higher in the high GS group than in the low and medium GS groups (P = 0.011, P < 0.001, P = 0.008, and P = 0.001, respectively), as shown in Table 4. Further, the lymphocyte count was significantly lower in the high GS group than in the other two groups (P = 0.001). In addition, DM is more prevalent in the high GS group than in the low and medium GS groups (35.6% vs. 23.1%, χ2 = 8.658, P = 0.003).
Table 4.
Comparison of diabetes mellitus and laboratory parameters between the high Gensini score group (GS > 60 points) and non-high Gensini score group (GS ≤ 60 points).
Univariate and multivariate analyses
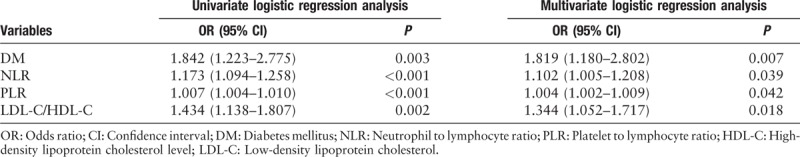
The risk factors of in-hospital MACEs and high GS were studied using univariate and multivariate logistic regression analyses. According to the univariate and multivariate logistic regression analysis [Tables 5 and 6], NLR, PLR, and GS were independent risk factors of in-hospital MACEs (odds ratio [OR]: 1.174, 95% confidential interval [CI]: 1.049–1.315, P = 0.005; OR: 1.012, 95% CI: 1.006–1.018, P < 0.001; and OR: 1.017, 95% CI: 1.009–1.025, P < 0.001, respectively). Further, the left main disease was also identified to be an independent and significant predictor of in-hospital MACEs (OR: 4.727, 95% CI: 1.514–14.759, P = 0.008). In addition, as shown in Table 7, the results of the multivariate logistic regression analysis of variables predicting a high GS (>60 points) suggested that NLR and PLR were also independent risk factors of severe CAD, assessed using the GS after adjusting for other factors, in patients with AMI (OR: 1.102, 95% CI: 1.005–1.208, P = 0.039 and OR: 1.004, 95% CI: 1.002–1.009, P = 0.042, respectively). Moreover, DM and LDL-C/HDL-C ratio were also identified to be independent risk factors of a high GS (OR: 1.819, 95% CI: 1.180–2.802, P = 0.007 and OR: 1.344, 95% CI: 1.052–1.717, P = 0.018, respectively).
Table 5.
Univariate logistic regression analysis of variables on in-hospital major adverse cardiovascular events.
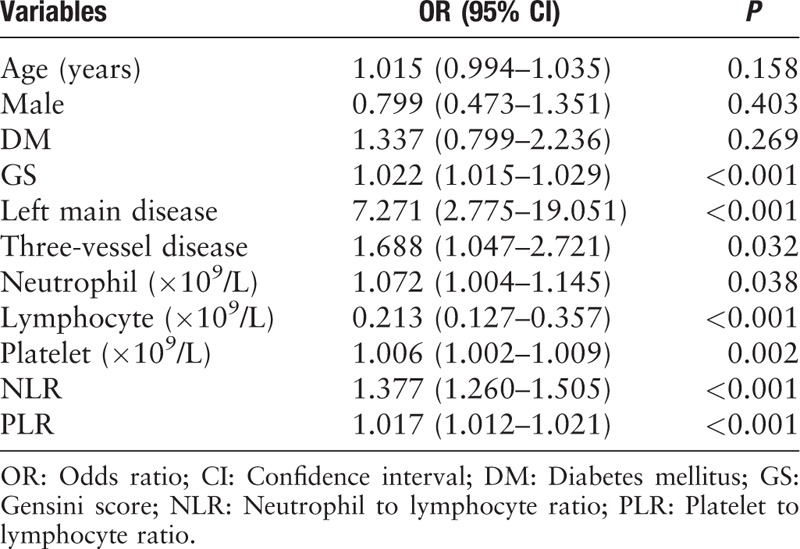
Table 6.
Multivariate logistic regression analysis of selected variables on in-hospital major adverse cardiovascular events.
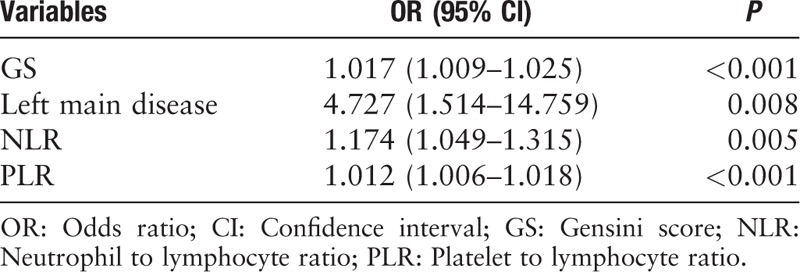
Table 7.
Univariate and multivariate logistic regression analysis of selected variables on a high Gensini score (>60 points).
ROC curve analysis
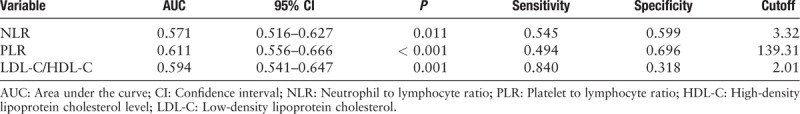
Figures 1 and 2 present the findings of the ROC curve analysis of NLR and PLR for predicting in-hospital MACEs and a high GS. The cutoff value of NLR for predicting the development of in-hospital MACEs was 5.05 with a sensitivity of 54.3% and a specificity of 82.4% (area under the curve [AUC]: 0.744, 95% CI: 0.685–0.803, P < 0.001). The cutoff value of PLR for predicting the occurrence of in-hospital MACEs was 151.28 with a sensitivity of 66.7% and specificity of 78.1% (AUC: 0.786, 95% CI: 0.730–0.842, P < 0.001). As shown in Table 8, an NLR of 3.32 was identified to be an effective cutoff point for detecting a high GS (>60 points) with a sensitivity of 54.5% and a specificity of 59.9% (AUC: 0.571, 95% CI: 0.516–0.627, P = 0.011), and a PLR of 139.31 was also identified to be a sufficient cutoff point for detecting a high GS (>60 points) with a sensitivity of 49.4% and a specificity of 69.6% (AUC: 0.611, 95% CI: 0.556–0.666, P < 0.001). Further, the cutoff value of LDL-C/HDL-C for predicting a high GS (>60 points) was 2.01 with a sensitivity of 84.0% and a specificity of 31.8% (AUC: 0.594, 95% CI: 0.541–0.647, P < 0.001).
Figure 1.
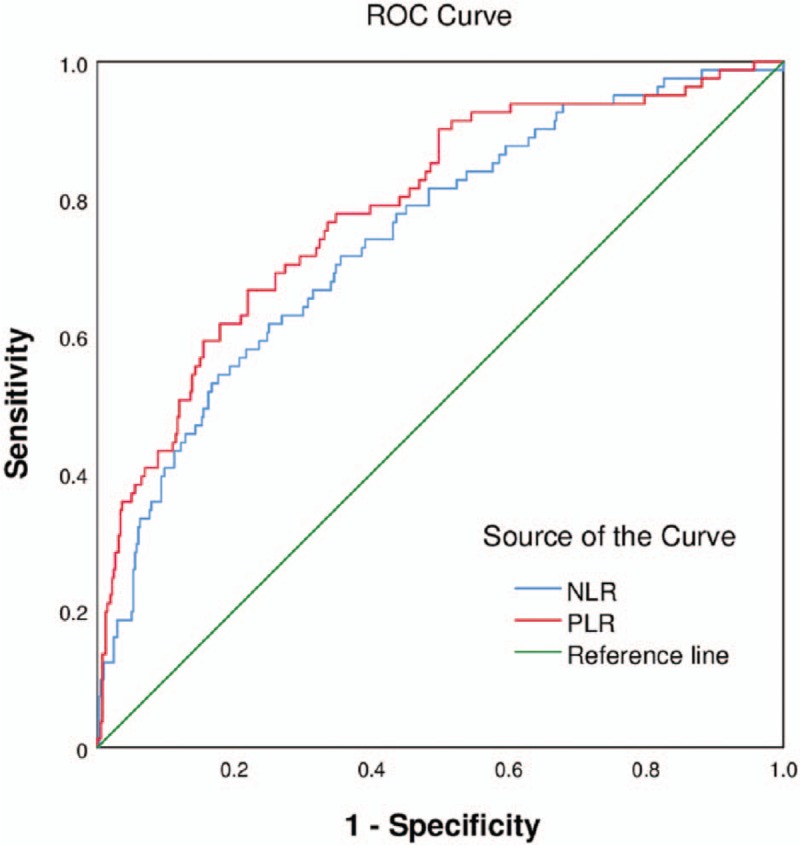
Receiver-operating characteristic (ROC) curve of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio for predicting in-hospital major adverse cardiovascular events. The ROC curve could assess neutrophil to lymphocyte ratio as a satisfying marker of in-hospital MACEs, yielding a cutoff of 5.05, with a sensitivity of 0.543 and a specificity of 0.824 (AUC: 0.744, 95% CI: 0.685–0.803, P < 0.001). Platelet to lymphocyte ratio yielding a cutoff of 151.28, with a sensitivity of 0.667 and a specificity of 0.781 (AUC: 0.786, 95% CI: 0.730–0.842, P < 0.001). MACEs: Major adverse cardiovascular events; AUC: Area under the curve; CI: Confidential interval.
Figure 2.
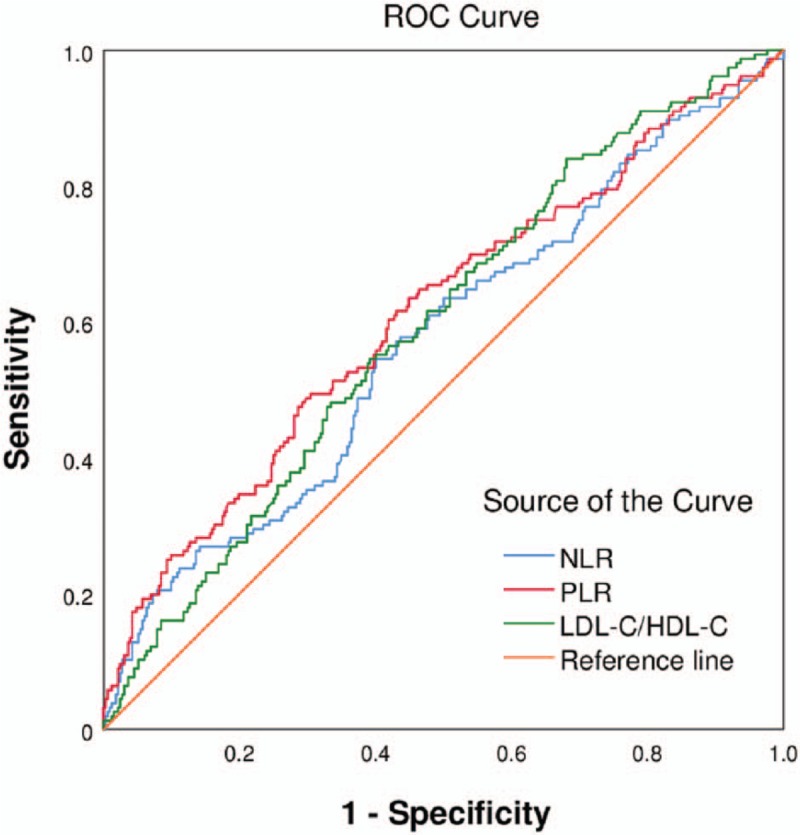
Receiver-operating characteristic (ROC) curve of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and LDL-C to HDL-C ratio for predicting a high Gensini score (>60 points). HDL-C: High-density lipoprotein cholesterol level; LDL-C: Low-density lipoprotein cholesterol.
Table 8.
Receiver-operating characteristic curve analysis of platelet to lymphocyte ratio and platelet to lymphocyte ratio and LDL-C to HDL-C ratio for predicting a high Gensini score (>60 points).
Discussion
Atherosclerotic CAD is the leading cause of mortality and morbidity worldwide. Atherosclerosis is a systemic, lipid-driven immune-inflammatory disease.[7] The inflammatory process plays a pivotal role in the initiation and development of atherosclerosis.[8] Furthermore, inflammation has also been reported to be one of the main causes of DM, hyperlipidemia, and metabolic syndrome.[9] Thus, various inflammatory biomarkers have emerged as potential predictors for identifying individuals at high risk for the unsatisfactory outcomes of CAD.
Various risk factors of coronary atherosclerosis can lead to injury of arterial intima and vascular endothelium and then result in the adhesion, aggregation, and activation of platelets. The present study has confirmed that platelets play a pivotal role in the pathophysiology of atherosclerotic CAD. Platelets compounded with fibrin lead to the formation of a coronary thrombus.[7] Furthermore, activated platelets could initiate and promote the development of atherosclerotic lesions by inducing endothelial cells and leukocytes to release inflammatory substances that cause monocyte adhesion and transmigration, and accelerating the recruitment of leukocytes in circulation to injured vascular endothelial cells.[10,11] These interactions between platelets, leukocytes, and endothelial cells together lead to the destabilization of atherosclerotic plaques. Gary et al[12] reported that higher platelet counts may lead to the elevation of blood viscosity and promote inflammation. Moreover, increased platelet count was also found to be involved in the formation of atherosclerotic plaques.[13] Kaplan et al[14] demonstrated that an elevated platelet count is associated with cardiovascular adverse events. The present study has shown that the platelet count is significantly related to the incidence of coronary restenosis and stent thrombosis.[15] In addition, platelet count has also been shown to be associated with short-term and long-term mortality in patients with ST-segment elevation myocardial infarction (STEMI), non-STEMI (NSTEMI), and unstable angina pectoris.[16,17]
Lymphocytes also play an important role in the atherosclerotic process,[18] which represents the regulatory pathway of the immune system. In AMI, lymphocytes infiltrate to the ischemic and reperfused myocardium and express interleukin-10, which may play an important role in the transmigration of mononuclear cells and induce the expression of tissue inhibitor of metalloproteinase-1.[19] Owing to the decrease of the total number of circulating plasma lymphocytes and the CD4/CD8 ratio in patients with the acute coronary syndrome (ACS), the body's anti-inflammatory ability declines significantly, leading to a short-term inflammatory status.[20] Ommen et al[21,22] reported that decreased lymphocyte count has been shown to be related to adverse events after AMI and advanced heart failure. In addition, low lymphocyte count has been reported to be associated with mechanical complications after myocardial infarction.[23]
Azab et al[2] found that PLR is associated with short-term and long-term mortality in patients after NSTEMI, and a similar relationship has also been reported for STEMI.[24] Thus, PLR is an important predictor of clinical cardiovascular events after AMI. Furthermore, PLR has also been demonstrated to be an independent risk factor for no-reflow, high SYNTAX scores, insufficient myocardial reperfusion, and in-hospital adverse events in patients with AMI undergoing primary PCI.[2,25] Kurtul et al[3] reported that in patients with ACS, PLR at admission is significantly associated with the severity and complexity of coronary atherosclerosis. And a high level of PLR may also be related to vulnerable plaque features of non-culprit lesions in patients with ACS.[26] PLR seems to be a significant predictor of the long-term outcomes of percutaneous interventions, and is of great importance in early risk stratification and providing timely intervention in patients with AMI.[27,28] In a prospective study performed by Lee et al[29] elevated PLR was verified to be associated with long-term all-cause mortality in patients at high risk of CAD who undergo coronary angiography. Li et al[30] revealed that high PLR was an independent factor associated with all-cause mortality and cardiovascular events in patients with ACS in a meta-analysis, and PLR was proved to be a promising biomarker in predicting worse prognosis in patients with ACS.[31]
The present study further demonstrated the role of high PLR as an independent risk predictor of in-hospital MACEs and CAD severity in AMI patients undergoing coronary angiography, suggesting that PLR could be used as a predictive marker for the assessment of high-risk AMI and CAD severity assessed by the GS. Moreover, owing to its characteristics of being inexpensive and broadly available in daily clinical practice, PLR could be useful as a supplemental marker to traditional risk factors for identifying high-risk patients with AMI and may contribute to guiding the evaluation and individualized targeted therapy of patients with AMI.
This study has several limitations. First, this was a single-center retrospective study with a small study population. Second, we did not strictly restrict the interval between the occurrence of AMI or the timing of admission, and we analyzed only the admission PLR, PLR may change dynamically with different clinical outcomes during the course of the disease, the lack of follow-up data of PLR is a notable drawback. Third, the severity of CAD should be evaluated by other clinical characteristics such as the plaque vulnerability at the same time instead of basing on the GS only. Multi-center prospective studies are warranted to assess the relationship between the dynamic changes of this biomarker and the long-term prognosis of AMI. Further, the combined effects of PLR with other traditional predictors should also be studied.
In summary, the present study demonstrated that, in patients with AMI, PLR is associated with in-hospital MACEs and CAD severity assessed according to the GS. Complete blood count analysis is a routine, inexpensive, and broadly available method that may be useful for the timely identification of high-risk patients. The combination of PLR and other traditional markers might be of great significance in identifying high-risk patients and providing timely intervention strategies to improve the prognosis of AMI.
Conflicts of interest
None.
Footnotes
How to cite this article: Li XT, Fang H, Li D, Xu FQ, Yang B, Zhang R, An Y. Association of platelet to lymphocyte ratio with in-hospital major adverse cardiovascular events and the severity of coronary artery disease assessed by the Gensini score in patients with acute myocardial infarction. Chin Med J 2020;133:415–423. doi: 10.1097/CM9.0000000000000650
References
- 1.Ozcan Cetin EH, Cetin MS, Aras D, Topaloglu S, Temizhan A, Kisacik HL, et al. Platelet to lymphocyte ratio as a prognostic marker of in-hospital and long-term major adverse cardiovascular events in ST-segment elevation myocardial infarction. Angiology 2016; 67:336–345. doi: 10.1177/0003319715591751. [DOI] [PubMed] [Google Scholar]
- 2.Azab B, Shah N, Akerman M, McGinn JT., Jr Value of platelet/lymphocyte ratio as a predictor of all-cause mortality after non-ST-elevation myocardial infarction. J Thromb Thrombolysis 2012; 34:326–334. doi: 10.1007/s11239-012-0718-6. [DOI] [PubMed] [Google Scholar]
- 3.Kurtul A, Murat SN, Yarlioglues M, Duran M, Ergun G, Acikgoz SK, et al. Association of platelet-to-lymphocyte ratio with severity and complexity of coronary artery disease in patients with acute coronary syndromes. Am J Cardiol 2014; 114:972–978. doi: 10.1016/j.amjcard.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Akboga MK, Canpolat U, Yayla C, Ozcan F, Ozeke O, Topaloglu S, et al. Association of platelet to lymphocyte ratio with inflammation and severity of coronary atherosclerosis in patients with stable coronary artery disease. Angiology 2016; 67:89–95. doi: 10.1177/0003319715583186. [DOI] [PubMed] [Google Scholar]
- 5.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 2018; 72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 6.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 1983; 51:606.doi: 10.1016/S0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 7.Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists’ view. Eur Heart J 2013; 34:719–728. doi: 10.1093/eurheartj/ehs411. [DOI] [PubMed] [Google Scholar]
- 8.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002; 105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 9.Balta S, Kurtoglu E, Kucuk U, Demirkol S, Ozturk C. Neutrophil-lymphocyte ratio as an important assessment tool. Expert Rev Cardiovasc Ther 2014; 12:537–538. doi: 10.1586/14779072.2014.902309. [DOI] [PubMed] [Google Scholar]
- 10.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest 2005; 115:3378–3384. doi: 10.1172/jci27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindemann S, Krämer B, Seizer P, Gawaz M. Platelets, inflammation and atherosclerosis. J Thromb Haemost 2007; 5:203–211. doi: 10.1111/j.1538-7836.2007.02517.x. [DOI] [PubMed] [Google Scholar]
- 12.Gary T, Pichler M, Belaj K, Hafner F, Gerger A, Froehlich H, et al. Platelet-to-lymphocyte ratio: a novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. PLoS One 2013; 8:e67688.doi: 10.1371/journal. pone.0067688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vidwan P, Lee S, Rossi JS, Stouffer GA. Relation of platelet count to bleeding and vascular complications in patients undergoing coronary angiography. Am J Cardiol 2010; 105:1219–1222. doi: 10.5114/aic.2017.66184. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan S, Kaplan ST, Kiris A, Gedikli O. Impact of initial platelet count on baseline angiographic finding and end-points in ST-elevation myocardial infarction referred for primary percutaneous coronary intervention. Int J Clin Exp Med 2014; 7:1064–1070. [PMC free article] [PubMed] [Google Scholar]
- 15.Nikolsky E, Grines CL, Cox DA, Garcia E, Tcheng JE, Sadeghi M, et al. Impact of baseline platelet count in patients undergoing primary percutaneous coronary intervention in acute myocardial infarction (from the CADILLAC trial). Am J Cardiol 2007; 99:1055–1061. doi: 10.1016/j.amjcard.2006.11.066. [DOI] [PubMed] [Google Scholar]
- 16.Ly HQ, Kirtane AJ, Murphy SA, Buros J, Cannon CP, Braunwald E, et al. Association of platelet counts on presentation and clinical outcomes in ST-elevation myocardial infarction (from the TIMI Trials). Am J Cardiol 2006; 98:1–5. doi: 10.11909/j.issn.1671-5411.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Mueller C, Neumann FJ, Hochholzer W, Trenk D, Zeller T, Perruchoud AP, et al. The impact of platelet count on mortality in unstable angina/non-ST-segment elevation myocardial infarction. Am Heart J 2006; 151:e1–7. doi: 10.1016/j.ahj.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Horne BD, Anderson JL, John JM, Weaver A, Bair TL, Jensen KR, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol 2005; 45:1638–1643. doi: 10.1016/j.jacc.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 19.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res 2002; 53:31–47. doi: 10.1016/S0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 20.Major AS, Fazio S, Linton MF. B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol 2002; 22:1892–1898. doi: 10.1161/01.atv.0000039169.47943.ee. [DOI] [PubMed] [Google Scholar]
- 21.Ommen SR, Gibbons RJ, Hodge DO, Thomson SP. Usefulness of the lymphocyte concentration as a prognostic marker in coronary artery disease. Am J Cardiol 1997; 79:812–814. doi: 10.1016/S0002-9149(96)00878-8. [DOI] [PubMed] [Google Scholar]
- 22.Ommen SR, Hodge DO, Rodeheffer RJ, McGregor CG, Thomson SP, Gibbons RJ. Predictive power of the relative lymphocyte count in patients with advanced heart failure. Circulation 1998; 97:19–22. doi: 10.1161/01.cir.97.1.19. [DOI] [PubMed] [Google Scholar]
- 23.Widmer A, Linka AZ, Attenhofer Jost CH, Buergi B, Brunner-La Rocca HP, Salomon F, et al. Mechanical complications after myocardial infarction reliably predicted using C-reactive protein levels and lymphocytopenia. Cardiology 2003; 99:25–31. doi: 10.1159/000068448. [DOI] [PubMed] [Google Scholar]
- 24.Sun XP, Li J, Zhu WW, Li DB, Chen H, Li HW, et al. Impact of platelet-to-lymphocyte ratio on clinical outcomes in patients with ST-segment elevation myocardial infarction. Angiology 2017; 68:346–353. doi: 10.1177/0003319716657258. [DOI] [PubMed] [Google Scholar]
- 25.Maimaiti A, Li Y, Wang YT, Yang X, Li XM, Yang YN, et al. Association of platelet-to-lymphocyte count ratio with myocardial reperfusion and major adverse events in patients with acute myocardial infarction: a two-centre retrospective cohort study. BMJ Open 2019; 9:e025628.doi: 10.1136/bmjopen-2018-025628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Xie Z, Liu X, Huang X, Lin J, Huang D, et al. Association of Platelet to lymphocyte ratio with non-culprit atherosclerotic plaque vulnerability in patients with acute coronary syndrome: an optical coherence tomography study. BMC Cardiovasc Disord 2017; 17:175.doi: 10.1186/s12872-017-0618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yildiz A, Yuksel M, Oylumlu M, Polat N, Akyuz A, Acet H, et al. The utility of the platelet-lymphocyte ratio for predicting no reflow in patients with ST-segment elevation myocardial infarction. Clin Appl Thromb Hemost 2015; 21:223–228. doi: 10.1177/1076029613519851. [DOI] [PubMed] [Google Scholar]
- 28.Vakili H, Shirazi M, Charkhkar M, Khaheshi I, Memaryan M, Naderian M. Correlation of platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio with thrombolysis in myocardial infarction frame count in ST-segment elevation myocardial infarction. Eur J Clin Invest 2017; 47:322–327. doi: 10.1111/eci.12736. [DOI] [PubMed] [Google Scholar]
- 29.Lee YSG, Baradi A, Peverelle M, Sultani R, Adams H, Garlick J, et al. Usefulness of platelet-to-lymphocyte ratio to predict long-term all-cause mortality in patients at high risk of coronary artery disease who underwent coronary angiography. Am J Cardiol 2018; 121:1021–1026. doi: 10.1016/j.amjcard.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Zhou Y, Ma Y, Han S, Zhou L. The prognostic value of the platelet-to-lymphocyte ratio in acute coronary syndrome: a systematic review and meta-analysis. Kardiol Pol 2017; 75:666–673. doi: 10.5603/KP.a2017.0068. [DOI] [PubMed] [Google Scholar]
- 31.Li W, Liu Q, Tang Y. Platelet to lymphocyte ratio in the prediction of adverse outcomes after acute coronary syndrome: a meta-analysis. Sci Rep 2017; 7:40426.doi: 10.1038/srep40426. [DOI] [PMC free article] [PubMed] [Google Scholar]


