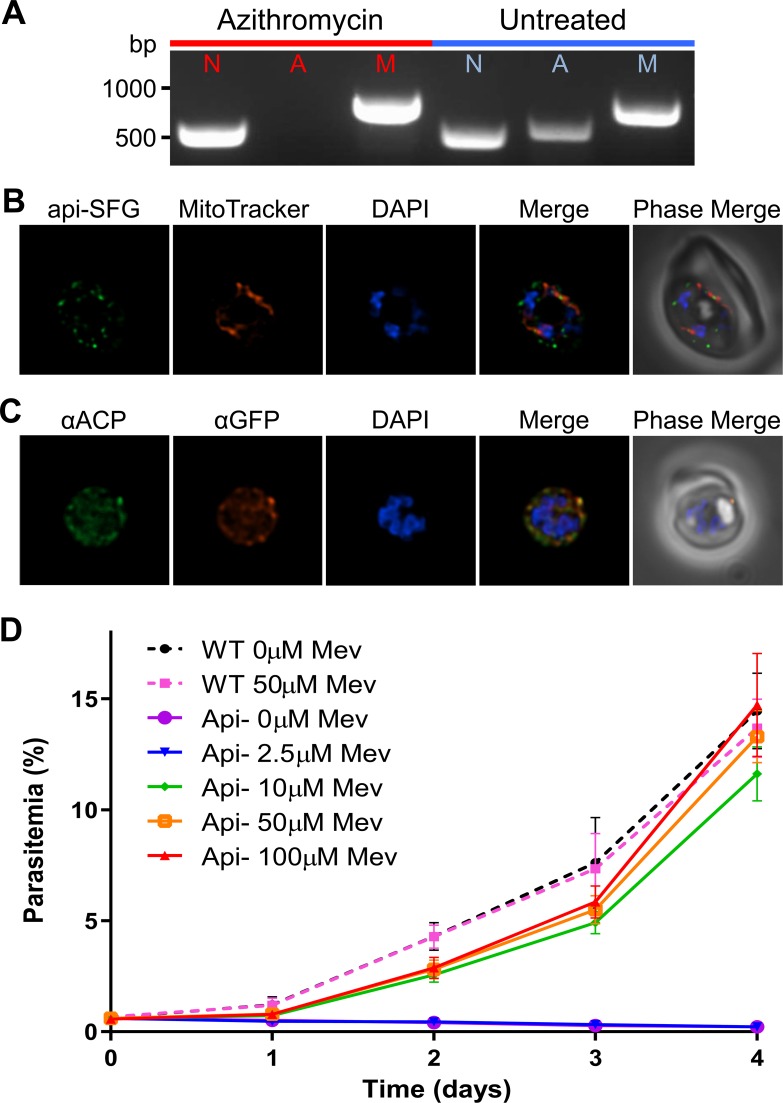
Fig 4. Generation and characterization of an apicoplast-disrupted PfMev parasite line.
A) Attempted detection of nuclear, mitochondrial, and apicoplast genomes by PCR amplification of the genes ldh (nuclear, N), sufB (apicoplast, A), and cox1 (mitochondrial, M) from PfMev parasites treated with 100nM azithromycin and 50μM mevalonate for one week (red), in addition to an untreated control (blue). Failure to amplify sufB in PfMev parasites treated with azithromycin/mevalonate indicates loss of the apicoplast organelle. B) Live fluorescence microscopy of PfMev parasites after treatment with 100nM azithromycin and 50μM mevalonate for one week, showing a disrupted organelle phenotype, with multiple discrete vesicles instead of a single intact organelle. The apicoplast is labeled with the api-SFG protein (green), the mitochondrion is stained with MitoTracker (red), and nuclear DNA is stained with DAPI (blue). C) Immunofluorescence co-localization of api-SFG with the apicoplast marker ACP, with αGFP (red), αACP (green), with nuclear DNA stained with DAPI (blue) in fixed apicoplast disrupted PfMev parasites. D) Growth curve of apicoplast-disrupted PfMev parasites grown in the presence of different concentrations of mevalonate. The growth of untreated (apicoplast intact) PfMev parasites was also measured in the presence of 50μM or 0μM mevalonate as a control. Supplementation with 10μM mevalonate, or more, restored the growth of apicoplast-disrupted parasites to near wild-type levels. Data from quadruplicate independent experiments, each conducted in quadruplicate are shown with error bars representing the standard error of the mean (SEM). Images depict fields that are 10 microns long by 10 microns wide.