Abstract
The jumonji domain‐containing protein 6 (JMJD6) is a Fe(II)‐ and 2‐oxoglutarate (2OG)‐dependent oxygenase that catalyses lysine hydroxylation and arginine demethylation of histone and non‐histone peptides. Recently, the intrinsic tyrosine kinase activity of JMJD6 has also been reported. The JMJD6 has been implicated in embryonic development, cellular proliferation and migration, self‐tolerance induction in the thymus, and adipocyte differentiation. Not surprisingly, abnormal expression of JMJD6 may contribute to the development of many diseases, such as neuropathic pain, foot‐and‐mouth disease, gestational diabetes mellitus, hepatitis C and various types of cancer. In the present review, we summarized the structure and functions of JMJD6, with particular emphasis on the role of JMJD6 in cancer progression.
Keywords: cancer, epigenetics, JMJD6 protein, jumonji domain‐containing histone demethylases
1. INTRODUCTION
The term “epigenetics” was first conceived by Conrad H. Waddington in the early 1940s to describe heritable changes in gene expression without alteration in DNA sequences.1 Deregulation of epigenetic processes leads to altered gene functions and a wide variety of pathologies, such as autoimmune diseases, metabolic diseases, neurological disorders and cancer.2, 3, 4 The key processes responsible for epigenetic regulation include DNA methylation, histone modification, nucleosome remodelling and alterations in non‐coding RNA profiles.1, 2 Histone methylation and hydroxylation, involving a wide range of epigenetic processes, have attracted considerable attention in the past decade.5
The jumonji C (JmjC) containing family of proteins are mainly composed of histone‐modifying enzymes, which are Fe(II)‐ and 2‐oxoglutarate (2OG)‐dependent oxygenases.6 The jumonji domain‐containing protein 6 (JMJD6), a member of the JmjC domain‐containing proteins, was originally identified as a phosphatidylserine receptor (PSR, Ptdsr) on cell surface.7 Subsequent studies demonstrated that JMJD6 is located in the nucleus, and has arginine demethylase and lysyl hydroxylase activities in histone and non‐histone proteins.8, 9 In addition, JMJD6 can also function as a tyrosine kinase of histone.10 There is growing evidence indicating the functions of JMJD6 at the transcriptional, splicing, posttranscriptional and biochemical levels, although the precise molecular mechanisms are not yet clear.8, 11, 12 JMJD6 promotes cell proliferation and migration in vitro, and accelerates tumour growth in vivo.13, 14 Overexpression of JMJD6 is correlated with advanced clinicopathological stage, increased aggressiveness and poor survival.12, 15, 16, 17
Here, we summarized the structure and functions of JMJD6 based on relevant basic researches. In particular, we focused on the role of JMJD6 in cancer progression and candidate mechanisms in order to highlight that JMJD6 may represent an attractive target for a new generation of anticancer drugs.
2. THE JMJD6 PROTEIN AND ITS STRUCTURE
2.1. From phosphatidylserine receptor to JMJD6
In 2000, JMJD6 was originally misassigned as a PSR expressed on the surface of macrophages, fibroblasts and epithelial cells.7, 18 Later studies reported the phosphatidylserine‐mediated clearance of apoptotic cells initiated by homologue of JMJD6 in Caenorhabditis elegans and zebrafish.19, 20 However, subsequent studies suggested that the function was incorrectly assigned and that the protein is predominantly located in cellular nucleus. In JMJD6‐deficient mice, the elimination of JMJD6 function leads to serious defects in the morphology of multiple organs and neonatal lethality, which cannot be explained by impaired apoptotic cell clearance.9 Therefore, JMJD6 was first demonstrated to be essential for the development, differentiation and maturation of multiple tissues during embryogenesis but not for apoptotic cells removal.9
In contrast to the proposed localization on cellular surface, a later study demonstrated that protein encoded by the JMJD6 cDNA is localized in the nucleus both in transfected cells and in cells expressing endogenous JMJD6 mRNA.21 Meanwhile, by cloning the homologous genes in Hydra, another study suggested that JMJD6 is a nuclear 2OG‐and Fe(II)‐dependent oxygenase that is capable of modifying nuclear proteins.22 Owning to this discovery and subsequent confirmation, the PSR was renamed to JMJD6.8, 22, 23, 24 JMJD6 exists in both cytoplasm and nucleus of MeWo cells; in the cytoplasm, JMJD6 presents as a soluble protein and associates with intracellular vesicles.25 JMJD6 has also been reported to be a secreted protein that can be detected in extracellular matrix.25
2.2. Structure of JMJD6
The results of sequence analysis demonstrated that JMJD6 contains a JmjC domain, which is highly conserved in proteins from eukaryotes to bacteria (Figure 1).21, 26, 27 The common core protein structural fold of all 2OG‐dependent oxygenases comprises the typical cupin or double‐stranded β‐helix fold (DSBH) (formed by 8 β‐strands within the JmjC domain) surrounded by characteristic secondary structure elements.28 Crystallographic studies confirmed the structure of DSBH; the DSBH fold forms a barrel‐like structure with two β‐sheets, and the Fe(II) binding site of the catalytic centre is located at the opening end of the barrel. The metal is most commonly bound by the side chain of three residues (His187, Asp189 and His273), which form an HXD/E(X)nH motif and are essential for the enzymatic activities of JMJD6.24, 29, 30, 31
Figure 1.
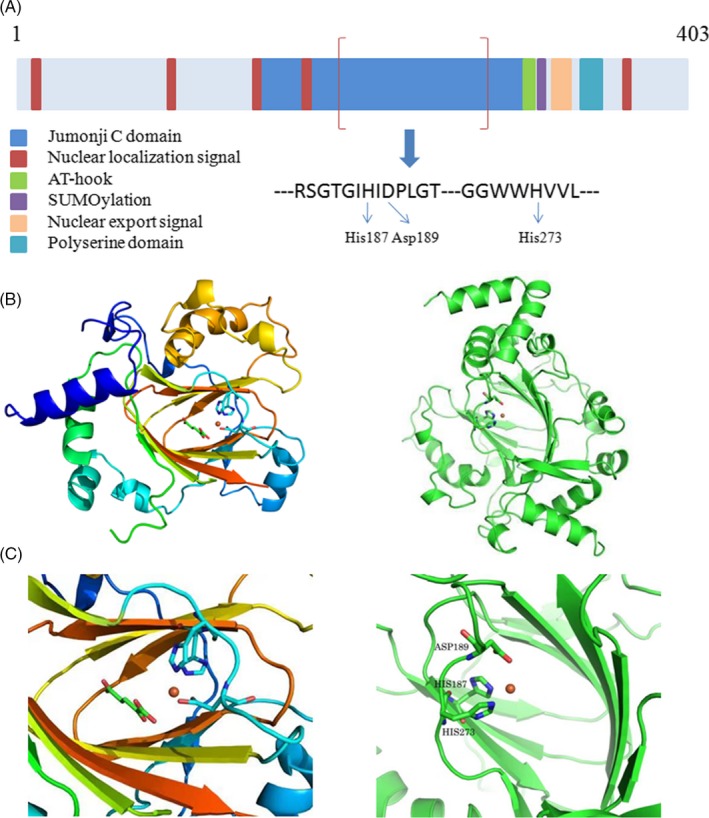
A, Diagram of the domain structure of Jumonji domain‐containing protein 6 (JMJD6). B, Three‐dimensional cartoon depicting the structure of JMJD6. C, The detailed structure of the HXD/E(X)nH motif in JmjC Domain, which is essential for the enzymatic activities of JMJD6. Conserved sequence motifs of JMJD6 protein include the following: a central Jumonji C domain (JmjC domain) (residues Pro141 to Gln286), five nuclear localization signals (NLSs), a DNA binding domain (AT‐hook) (residues Lys300 to Ser309), a putative SUMOylation site (Leu316 to Glu319) and a polyserine (polyS) region. His187, Asp189 and His273 are Fe (II) complexing residues
In addition to the JmjC domain, five nuclear localization signals (NLSs), a nuclear export signal (NES), a DNA binding domain (AT‐hook) (residues Lys300 to Ser309) and a putative SUMOylation site (Leu316 to Glu319) are the conserved sequence motifs of JMJD6 protein (Figure 1).22, 26, 27, 32 Examinations of JMJD6 amino acid sequence showed the presence of five functional NLSs that can target JMJD6 to the nuclei either alone or in concert.21, 22 Two of the five NLSs overlap with the JmjC domain and may not be topologically accessible in vivo.21 The AT‐hook was initially described as a DNA binding motif; however, JMJD6 binds efficiently to single‐stranded RNA, but does not bind to DNA.33, 34, 35 JMJD6 was suggested to be a type of non‐canonical AT‐hook‐like domain protein.35 Using the CBS‐prediction service, a putative SUMOylation site (probability score 92%) was identified in the JMJD6 protein, and the SUMOylation site might be used to regulate its interactions with other proteins.26 Furthermore, three‐dimensional structural model of JMJD6 protein indicates that the NLSs, the AT‐hook and the SUMOylation site may be accessible for interacting proteins.26
Jumonji domain‐containing protein 6 has a polyserine (polyS) region at its C‐terminus. The polyS region is highly conserved and comprises 16 serine residues interrupted by 4 aspartate residues (Ser340‐Ser359) (Figure 1).32 This C‐terminal polyS is missing in JMJD6 splice variants.26 In some bacterial extracellular modular carbohydrate degrading enzymes, the polyS region was suggested to be a flexible linker connecting substrate and binding enzymatic domains.36 A cell‐based study demonstrated that the polyS region of JMJD6 protein participates in the bidirectional nucleoplasmic‐nucleolar shuttling of JMJD6.32 The presence/absence of the polyS region regulates the subnuclear localization of JMJD6 protein. In the JMJD6 protein, the polyS domain may have a regulatory influence on its oligomeric structure. Transmission electron microscopy (TEM) studies indicated that the structure of JMJD6 oligomer depends on the presence of the polyS domain; JMJD6 lacking the polyS domain forms a filamentous structure, while JMJD6 with complete polyS domain forms a ring‐shaped overall oligomeric structure.32
JMJD6 can exist in monomeric and larger oligomeric forms.37 Several studies showed that JMJD6 adopts an oligomeric form in solution. However, by Western blot analysis of full‐length recombinant JMJD6, both monomeric and oligomeric forms were detected in solution, and the oligomers correspond to apparent trimeric, pentameric and larger oligomeric forms.31, 38, 39, 40 The existence of JMJD6 oligomerizes in cells was confirmed by co‐immunoprecipitation and fluorescence two‐hybrid assays.32, 40
3. THE ENZYMATIC ACTIVITIES OF JMJD6
So far, the enzymatic activities are considered to be the most important characteristics of JMJD6. JMJD6 has been reported to possess arginine demethylase and lysyl hydroxylase activities for a long time (Figure 2A,B). Recently, the intrinsic tyrosine kinase activity of JMJD6 was also observed. JMJD6 phosphorylates Y39 of histone H2A.X using both guanosine triphosphate (GTP) and adenosine triphosphate (ATP) as phosphate donors and regulates the expression of autophagy‐related genes (Figure 2C).10 Unlike any known kinase protein, JmjC domain and the polyS domain are required for the kinase function of JMJD6. Overall, JMJD6 is likely to have many potential catalytic activities, but the long‐standing controversies surrounding the enzymatic spectrum of JMJD6 should be addressed through more in‐depth experiments. Here, we mainly summarized the two enzymatic actions of JMJD6: hydroxylation and demethylation.
Figure 2.
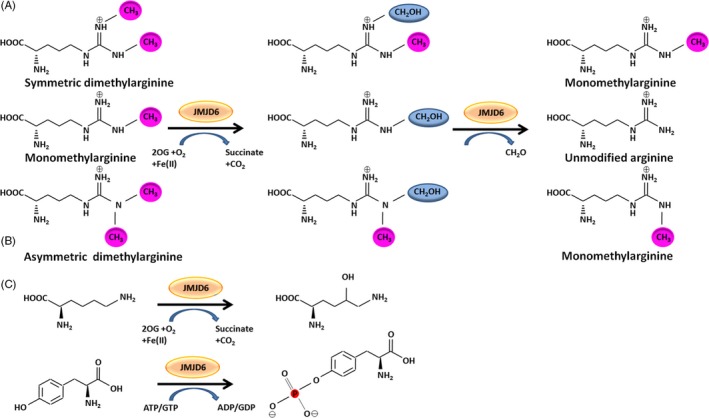
JMJD6 functions as arginine demethylase (A), lysyl hydroxylase (B) and tyrosine kinase (C). A, Demethylation reactions of symmetric dimethylarginine, monomethylarginine and asymmetric dimethylarginine catalysed by JMJD6. In the first step, JMJD6 hydroxylates the methyl group consuming oxoglutarate (2OG), and in the second step, a deformylation reaction produces formaldehyde (CH2O) to form an unmodified arginine. B, JMJD6 catalyses lysine hydroxylation. C, JMJD6 phosphorylates tyrosine using both guanosine triphosphate (GTP) and adenosine triphosphate (ATP) as phosphate donors
3.1. JMJD6 as an arginine demethylase
One of the proposed functions of JMJD6 is catalysing arginine demethylation (Figure 2A). This reaction depends on the presence of cofactors, including Fe (II) and 2OG.37 Arginine methylation occurs in many proteins involved in various cellular functions. Among them, histones have long been known to be substrates for methylation.41 Methylation of histone plays an important role in the regulation of transcription, genome integrity and epigenetics.5 In mammals, methylation of histone arginine is typically found on several residues, including residues 2, 8, 17 and 26 of histone H3 (H3R2, H3R8, H3R17, H3R26) and residue 3 of histone H4 (H4R3).41, 42 In 2007, JMJD6 was first experimentally demonstrated to function as a dioxygenase.8 Notably, JMJD6 demethylates monomethylarginine and symmetric and asymmetric dimethylarginine residues (Figure 2A). Recent studies found that JMJD6 also targets arginine residues of non‐histone proteins for demethylation, including RNA helicase A, oestrogen receptor α (ERα), tumour necrosis factor receptor‐associated factor 6 (TRAF6), the transcription factor PAX3 and heat‐shock protein 70 (HSP70).43, 44, 45, 46, 47, 48
The role of JMJD6 as a histone arginine demethylase remains controversial. On the one hand, evidence that JMJD6 directly demethylates proteins is still absent, and therefore, we cannot rule out that JMJD6 may indirectly affect the demethylation of these proteins. On the other hand, several studies reported that histone arginine demethylation activity of JMJD6 was not observed in their study.23, 49, 50 JMJD6 was incubated with arginine–serine‐rich (RS) domain (arginine‐rich sequences present in this domains) in the presence of oxygen, Fe(II) and 2OG, and subsequently analysed by means of matrix‐assisted laser desorption/ionization (MALDI) mass spectrometry (MS).23 The results showed that JMJD6 cannot produce demethylated arginine histone H4 and H3 fragment peptides.23 In another study, JMJD6 silencing in endothelial cells was shown to not affect arginine methylation at H4R3.50 In addition, a study on the crystal structure of JMJD6 also doubts on its ability to demethylate arginine residues based on the structural data.49 Even so, arginine demethylation catalysed by JMJD6 cannot be ruled out and further studies are expected.
3.2. JMJD6 as a lysyl hydroxylase
In addition to its arginine demethylase activity, JMJD6 also has strong lysyl hydroxylase activity (Figure 2B).23, 51, 52 After incubation of U2 small nuclear ribonucleoprotein auxiliary factor 65‐kilodalton subunit (U2AF65) with JMJD6, 2OG and iron, the results of liquid chromatography‐mass spectrometry (LC‐MS)/MS analysis showed that U2AF65 is a substrate of JMJD6 and JMJD6 executes lysine‐specific hydroxylation of U2AF65 (from HeLa cells) at lysine K15 (hydroxylated:unhydroxylated, 1:100) and K276 (hydroxylated:unhydroxylated, 1:250) residues.23 Moreover, in HeLa cells, JMJD6 overexpression results in increased hydroxylation of U2AF65.23 No evidence of hydroxylation of lysyl residues in endogenous histones (H2A, H2B, H3 and H4) was accrued in this study.23 However, in 2013, another study developed an alternative method, namely amino acid composition analysis, to detect 5‐hydroxylation of histone lysyl residues.51 This study reported that JMJD6 can hydroxylate multiple lysine residues of histone H3 and H4.51 It indicates that in addition to the only known lysyl hydroxylases, the procollagen lysyl hydroxylase (PLOD enzymes), JMJD6 also functions as a specialized lysyl hydroxylase.
3.3. Regulation of JMJD6 functions
The activity of JMJD6 is regulated by hypoxia, iron and 2OG availability.31 The upregulation of JMJD6 can be induced by hypoxia.53 The hypoxia‐inducible factor (HIF) hydroxylases are the major regulators of the hypoxia response, probably in animals ranging from nematode worms to man.54 Hypoxia can upregulate metastasis‐associated lung adenocarcinoma transcript 1 (MALAT1), a long non‐coding RNA associated with cancer progression and metastasis.55 JMJD6 can be positively regulated by MALAT1 through MALAT1/miR‐125/JMJD6 axis. Furthermore, tricarboxylic acid (TCA) cycle intermediates, including succinate, fumarate and succinate, can inhibit the activities of 2OG oxygenases.48, 56
4. JMJD6 IN CANCER PROGRESSION
Abnormal expression of JMJD6 may contribute to the development of many diseases, such as neuropathic pain, foot‐and‐mouth disease, gestational diabetes mellitus, hepatitis C and various types of cancer.12, 57, 58, 59, 60, 61 Here, we summarized the role of JMJD6 in the progression of several types of cancer (Table 1).
Table 1.
The role of JMJD6 in different types of cancer
| Cancer type | Findings from in vitro and in vivo studies | Findings from clinical data | References |
|---|---|---|---|
| Breast cancer | 10, 12, 14, 23, 62 | ||
| ER− breast cancer | JMJD6 is associated with increased cell proliferation, migration, invasion and metastases |
JMJD6 expression is positively correlated with histological grade, age, LN metastasis, tumour size and advanced TNM stage. High level of JMJD6 may/not indicates poor survival (different conclusions are reported) |
|
| ER+ breast cancer | JMJD6 promotes/inhibits proliferation and migration of MCF‐7 cells (contradictory conclusions are reported) |
Expression of JMJD6 in ER+ tumours is slightly but significantly lower than ER− tumours. JMJD6 is highly expressed in more aggressive and advanced tumours. High JMJD6 expressers have poorer outcomes than low expressers |
|
| Melanoma |
JMJD6 facilitates proliferation and invasion of melanoma cells in vitro, and promotes growth and metastasis of melanoma in vivo. JMJD6 enhances blood vessel formation |
JMJD6 expression is increased in melanoma. At later stages of melanoma progression, JMJD6 level is elevated. High level of JMJD6 is associated with unfavourable prognosis |
63, 64 |
| Oral cancer |
JMJD6 is enriched in cancer stem cells. JMJD6 promotes cancer stem cell properties, and knock‐down of JMJD6 suppresses stem‐like property of OSCC |
Expression level of JMJD6 is higher in carcinoma tissues than in normal tissues | 66 |
| Lung adenocarcinoma | Acetylation of HOXB9 at lysine 27 decreases its ability to promote the migration and growth of lung cancer cells in mice through suppressing the transcription of JMJD6 |
JMJD6 mRNA and protein are significantly increased in human lung adenocarcinoma specimens. The level of JMJD6 is significantly associated with clinical parameters. The survival of patients with high JMJD6 expression is poorer than those with low JMJD6 expression |
71, 72 |
| Glioblastoma | Inhibiting JMJD6 with shRNA could improve survival in the orthotopic xenograft mouse model of glioblastoma, but could not alter cell growth and survival in vitro | JMJD6 mRNA and protein are significantly elevated in human gliomas tissues and are increased with tumour grade | 73, 74 |
| Hepatocellular carcinoma |
JMJD6 promotes proliferation and migration of HCC cell lines. JMJD6 regulates cell cycle and apoptosis progression of HCC cell lines |
JMJD6 is significantly correlated with tumour grade and TNM stage. High JMJD6 expression in tumour tissue is indicative of poor prognosis |
17 |
| Colon carcinoma |
JMJD6 increases the percentage of cells in the G1 phase, promotes cell apoptosis and sensitizes cells to DNA damaging agents. JMJD6 promotes cellular proliferation and tumorigenesis in vivo |
JMJD6 protein is significantly increased in colon adenocarcinomas. High level of JMJD6 expression is correlated with increased invasiveness, poor differentiation, lymph node metastases and advanced stage. JMJD6 is associated with worse prognosis |
16 |
| Ovarian cancer |
Inhibition of JMJD6 suppresses cellular proliferation and migration, and promotes apoptosis of ovarian cancer cell lines. JMJD6 inhibitor exhibits powerful therapeutic effects on ovarian cancer in vivo |
JMJD6 is highly expressed in 61.64% of 146 ovarian cancer patients. High expression of JMJD6 is significantly associated with age, clinical stage, pT status and pN status of the ovarian cancer patients. High level of JMJD6 indicates high risk of disease progression and death |
75 |
| Neuroglioma |
JMJD6 expression is increased notably in neuroglioma stem cells than other neuroglioma cells. JMJD6 promotes proliferation, migration and invasion of neuroglioma stem cells |
— | 13 |
Abbreviations: HCC, hepatocellular carcinoma; JMJD6, jumonji domain‐containing protein 6; LN, lymph node; OSCC, oral squamous cell carcinoma; pN status, pathological node status; pT status, pathological tumour status; TNBC, triple‐negative breast cancer; TNM, tumour node metastasis.
4.1. Breast cancer
In triple‐negative breast cancer cell lines, oestrogen‐induced breast cancer cells and MMTV‐Myc mammary tumour cells, in vitro and in vivo experiments indicated that high level of JMJD6 leads to increased cell proliferation, migration, invasion and metastases.12, 62 In MCF7 breast cancer cell line, Poulard et al reported that JMJD6 promotes proliferation and migration in vitro and tumour growth in vivo, whereas Lee et al reported opposite findings.14 Combined inhibition of JMJD6 kinase activity and autophagy is an effective therapeutic strategy for triple‐negative breast cancer.10 In mice injected with breast carcinoma cells, treatment with P4E11, a monoclonal antibody specific for JMJD6, can reduce fibrosis at the primary tumour and metastatic burden by blockading the interaction of JMJD6 with collagen I, which is also confirmed in xenograft mouse model of ovarian cancer.25
In breast cancer samples from patients, the expression of JMJD6 is different in different breast cancer subtypes: JMJD6 expression in ER‐positive (ER+) tumours is slightly but significantly lower than in ER‐negative (ER−) tumours (JMJD6 expression is consistently associated with ER− disease); the expression of JMJD6 is highest in Claudin‐low and basal subtypes followed by HER2‐enriched and luminal B subtypes, and lowest in luminal A subtype.12 In both ER+ and ER− breast cancer patients, elevated expression of JMJD6 is positively associated with histological grade, age, lymph node metastasis, tumour size and advanced tumour node metastasis (TNM).12, 62 Unfavourable survival was observed in high JMJD6 expressers with ER+ breast cancer.10, 62 However, in ER− breast cancer patients, several studies have reported that there is no significant correlation between JMJD6 level and survival, probably due to the high level of JMJD6 expression in ER− breast cancer.12, 62 Furthermore, the expressions of JMJD6 in cancer tissues and paired adjacent tissues are different in different breast cancer subtypes; JMJD6 levels in cancer tissues are higher than in adjacent matched tissues in 90% patients with triple‐negative breast cancer, but this ratio is 10% in patients with other breast cancer subtypes. Taken together, the role of JMJD6 in promoting breast cancer progression has been established, although its role in different subtypes of breast cancer may not be identical.
4.2. Melanoma
Jumonji domain‐containing protein 6 regulates melanogenesis in melanoma cells because overexpression of JMJD6 promotes the expression of microphthalmia‐associated transcription factor (MITF), a master regulator of melanogenesis.63 JMJD6 facilitates multiple cellular processes, including proliferation and invasion of melanoma cells in vitro, and promotes growth and metastasis of melanoma in vivo.63 At later stages of melanoma development in zebrafish, the expression of JMJD6 is elevated.64 Furthermore, JMJD6 is capable of enhancing blood vessel formation in melanoma.63
In human melanoma tissues, JMJD6 expressions were increased in both primary and metastatic melanomas than normal tissues, with higher expression of JMJD6 in metastatic melanoma.63 JMJD6 is closely correlated with lymph node involvement, distant metastases and more aggressive phenotypes, whereas depth of invasion is not correlated with the expression of JMJD6.63 Compared with patients with wild‐type JMJD6, patients with mutation, amplification, deep deletion or multiple alteration of JMJD6 have an unfavourable prognosis.64 Collectively, JMJD6 plays an important role in the development and progression of melanoma.
4.3. Oral cancer
Cancer stem cells comprise a small population of cells within a tumour and are responsible for initiation and long‐term sustenance of cancer.65 Cancer stem cells are considered as the root of cancer owing to their important role in tumorigenesis, tumour metastasis and tumour recurrence.66 JMJD6 is enriched in cancer stem cells, and knock‐down of JMJD6 suppresses the tumour sphere formation (a characteristic of cancer stem cells in human cancer cells) of tested cell lines, indicating that JMJD6 is required for the stem‐like properties of oral squamous cell carcinoma.66, 67, 68 Furthermore, overexpression of JMJD6 promotes cancer stem cell properties, including self‐renewal capacity, migration ability and resistance to chemotherapy.66, 69, 70 The expression of JMJD6 in oral squamous cell carcinoma cell lines is higher than that in precancerous cell lines, and it also positively correlates with the development of squamous cell carcinoma.66
Immunohistochemical staining of 18 normal human oral epithelia samples and 16 oral squamous cell carcinoma samples showed that the expression level of JMJD6 is higher in carcinoma tissues. In oral squamous cell carcinoma cases, the strong JMJD6 staining rate is 69%, while 89% normal human oral epithelia cases show weak JMJD6 staining. Therefore, JMJD6 plays a role in the development of oral cancer, in part because it serves as a molecular determinant of cancer stem cell phenotype.
4.4. Lung adenocarcinoma
Jumonji domain‐containing protein 6 mRNA and protein are significantly increased in human lung adenocarcinoma specimens than in corresponding non‐tumour lung tissues.71, 72 The level of JMJD6 is significantly associated with clinical parameters, such as tumour size, pathological grade, pathological tumour (pT) status, pathological node (pN) status and pleural invasion.71 In the overall survival of lung adenocarcinoma patients, JMJD6 plays a negative role.71, 72 Furthermore, the results of in vitro experiments indicated that acetylation of HOXB9 at lysine 27 decreases its ability to promote migration and growth of lung cancer cells in mice through suppressing the transcription of JMJD6, supporting that JMJD6 indeed acts as an oncogenic protein.72
4.5. Glioblastoma
Levels of JMJD6 mRNA and protein are significantly elevated in human glioma tissues, and are increased with tumour grade. Inhibiting JMJD6 with short hairpin RNA (shRNA) or single‐guide RNA (sgRNA) could not alter cell growth, colony formation and survival in vitro.73 In the orthotopic xenograft mouse model, targeting JMJD6 is of great benefits to survival, and sustained JMJD6 inhibition may provide even better anti‐tumour effects.73 In addition, compared with mice implanted with normal glioblastoma cells, the survival of mice bearing JMJD6‐deficient glioblastoma cells is significant improved. Therefore, these findings provide evidence that JMJD6 plays a key role in glioblastoma and may be a potential therapeutic target of glioblastoma.73, 74
4.6. Hepatocellular carcinoma
Knock‐down of JMJD6 reduces the migratory ability, proliferation rate and colony formation of hepatocellular carcinoma cell lines.17 The results of flow cytometry analyses showed that JMJD6 increases the proportion of cells in the S phase, reduces the proportion of cells in the G1 phase.17 Overexpression of JMJD6 reduces the apoptosis of human hepatoma‐derived cell lines.17 The JMJD6 expression level is significantly increased in human hepatocellular carcinoma tissues than in normal liver tissues.17 JMJD6 protein level in hepatocellular carcinoma is positively correlated with histological grade, and the JMJD6 mRNA level is significantly correlated with tumour grade and TNM stage.17 In addition, high JMJD6 expression in tumour tissues is indicative of poor prognosis.17
4.7. Colon carcinoma
Jumonji domain‐containing protein six knock‐down in colon cancer cell lines increases the percentage of cells in the G1 phase, reduces the cell population in the S phase, promotes cell apoptosis and sensitizes cells to DNA damaging agents.16 The results of xenograft experiments performed in mice showed that JMJD6 promotes cellular proliferation and tumour growth.16 Immunohistochemical staining of 90 colon carcinoma samples with paired adjacent normal tissues showed that JMJD6 protein is significantly increased in colon adenocarcinoma.16 High level of JMJD6 expression is correlated with increased invasiveness, poor differentiation, lymph node metastasis and advanced stage.16 In addition, follow‐up data showed that elevated JMJD6 is associated with worse outcomes of patients with colon carcinoma.16
4.8. Ovarian cancer
Inhibition of JMJD6 with compound 2‐(2‐(2‐hydroxybenzylidene) hydrazinyl)‐6‐methylpyrimidin‐4‐ol (termed SKLB325) inhibits cellular proliferation and migration, and promotes apoptosis of ovarian cancer cell lines.75 In the intraperitoneal xenograft model, JMJD6 inhibitor exhibits powerful therapeutic effects on ovarian cancer.75 Among 146 patients with ovarian cancer, JMJD6 is highly expressed in 61.64% of them.75 High expression of JMJD6 is significantly associated with age, clinical stage, pT status and pN status of the patients.75 In patients with serous and mucinous ovarian cancer, high level of JMJD6 indicates high incidence of disease progression and death.75
4.9. Neuroglioma
In a study of neuroglioma, neuroglioma stem cells with self‐renew and multipotential differentiation ability were isolated.13 The results of Western blot indicated that JMJD6 expression is increased notably in neuroglioma stem cells than in other neuroglioma cells.13 Furthermore, JMJD6 promotes proliferation, migration and invasion of neuroglioma stem cells.13
5. MECHANISMS OF JMJD6 IN PROMOTING CANCER DEVELOPMENT
Although the mechanisms through which JMJD6 promotes cancer progression remain unclear, several mechanisms have been proposed in published studies. Here, we summarized the important candidate mechanisms (Figure 3).
Figure 3.
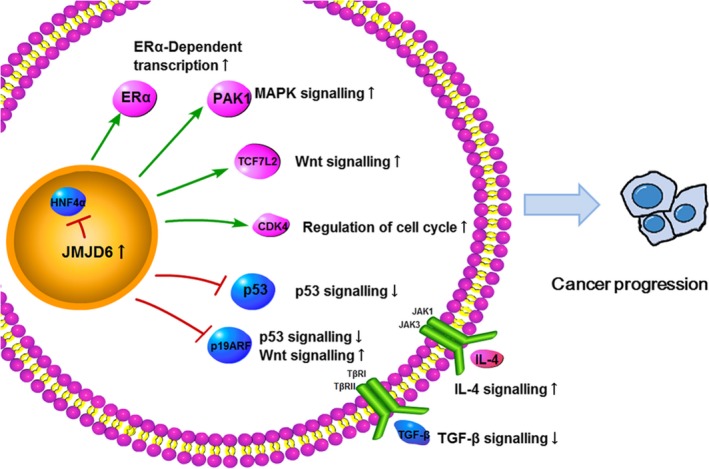
The candidate mechanisms through which JMJD6 promotes cancer progression: JMJD6 cooperates with cancer‐promoting signalling through interacting with ERα, PAK1, TCF7L2, CDK4 and IL‐4 (pink), and represses the cancer suppression signalling through regulating HNF4α, p53, p19ARF and TGF‐β (blue)
5.1. JMJD6 regulates cancer‐related signalling
5.1.1. JMJD6 downregulates p53 activity
Jumonji domain‐containing protein 6 has been reported to interact with p53 and participate in its posttranslational modification.16 Perturbations in p53 signalling pathways are thought to play an important role in the development of cancer, and mutations leading to loss of wild‐type p53 activity are often detected in many different types of cancer.76 In human colon carcinoma HCT116 cells, JMJD6 is physically associated with the tumour suppressor p53, and the C‐terminal fragment of p53 (from residues 290 to 393) is required for the binding of p53 to JMJD6. Although hydroxylation of p53 protein has not been reported before this study, it was demonstrated that JMJD6 acts as a 2‐OG‐ and Fe(II)‐dependent lysyl hydroxylase and catalyses hydroxylation of p53 on lysine 382 (K382). Furthermore, elevated expression of JMJD6 in cells leads to an increase in the amount of K382 hydroxylation of p53. HCT116 cells depleted of JMJD6 by siRNA showed increased levels of both mRNA and protein of p21 and PUMA, two well‐characterized p53 downstream target genes. JMJD6 knock‐down arrests cells in the G1 phase, induces cell apoptosis, makes cells sensitive to DNA damaging agents and represses p53‐dependent colon cell proliferation and tumour development in a p53‐dependent manner. JMJD6 negatively regulates p53 transcriptional activity through hydroxylation modification. In addition to being hydroxylated, lysine 382 of p53 can also be acetylated by the acetyl transferase p300/CBP, which has been reported to enhance the transcriptional activity of p53. However, hydroxylation of lysine 382 antagonizes p300/CBP‐mediated acetylation. Together, these findings suggested that JMJD6 catalyses hydroxylation of p53 and downregulates its transcriptional activity, thereby inhibiting the tumour suppressor function of p53.
5.1.2. JMJD6 upregulates Wnt signalling
Since Wnt signalling is critical for the activity of epithelial stem cells, it is not surprising that Wnt signalling is frequently upregulated in cancer.77 In glioma stem cells, cignal finder cancer 10‐pathway reporter array was adopted to explore the signalling pathways involved in the association between JMJD6 and increased cell proliferation, migration and invasion.13 The results demonstrated that silencing of JMJD6 with JMJD6‐shRNA suppresses Wnt signalling and activates p53 signalling. Further studies measured the expression of essential molecule T‐cell factor/lymphoid enhancer factor (TCF/LEF) family protein Tcf7l2 in Wnt signalling and found that silencing of JMJD6 significantly reduces the Tcf7l2 expression.13 JMJD6 has also been demonstrated to interact with and depress TCF/LEF family protein Tcf7l1 (also known as Tcf3), a transcriptional repressor that inhibits transcription of Wnt target genes by recruiting Groucho‐related transcriptional corepressors.78, 79 This study then showed that JMJD6 does enhance Wnt signalling. The aa 33‐410 region of Tcf7l1 is the binding domain between Tcf7l1 and JMJD6, which is also responsible for the interaction of Tcf7l1 with Groucho.79 Moreover, miR‐770 binds to the 3′UTR of JMJD6 mRNA and suppresses its expression, resulting in the downregulation of WNT/β‐catenin pathway, thereby exerting anti‐tumour effect.80
5.1.3. JMJD6 upregulates MAPK signalling
The mitogen‐activated protein kinase (MAPK) cascade is a critical pathway for human cancer cell survival, migration and resistance to drug therapy.81, 82, 83 RNA deep‐sequencing and bioinformatics analyses indicated that silencing of JMJD6 with siRNAs affects the alternative splicing key components of the MAPK signalling pathway, such as PAK1 (p21‐activated kinase 1), RAPGEF2 and MAP3K4.63 Among these, PAK1 is capable of directly phosphorylating RAF and MEK1 (mitogen‐activated protein kinase kinase 1), thereby positively regulating MAPK signalling.63, 84, 85 Further studies demonstrated that JMJD6 binds to PAK1 precursor messenger RNA (pre‐mRNA) and affects the alternative splicing of PAK1 through promoting exon inclusion and generation of the full‐length PAK1.63 Interestingly, MAPK signalling is also associated with JMJD6 expression, because of that downregulation of MAPK signalling contributes to reduced mRNA and protein levels of JMJD6.63 It was suggested that hyperactive MAPK signalling leads to the phosphorylation of c‐Jun, and then, activated c‐Jun transactivates JMJD6.63 In summary, there may be a feedforward regulatory loop between JMJD6 and the MAPK signalling pathway.
5.1.4. JMJD6 suppresses Myc‐induced apoptosis
Myc expression is commonly deregulated in many cancers of different origins. Myc plays an important role in multiple cellular processes that promote survival of cancer cells.86, 87 To curb cell cycle progression in response to increased Myc, increased Myc also induces p19ARF expression, thereby leading to cell apoptosis through the activation of p53.88, 89 Furthermore, p19ARF binds with Myc and prevents Myc‐mediated tumorigenesis in a p53‐independent manner.89 JMJD6 cooperates with Myc to enhance tumorigenesis through suppressing Myc‐induced apoptosis.62 JMJD6 binds to the p19ARF promoter and demethylates Arg3 of histone H4, thereby repressing p19ARF and reducing p53 levels. Moreover, JMJD6 overexpression can induce epithelial‐mesenchymal transformation and greatly enhance tumour growth and invasion.62
5.1.5. JMJD6 suppresses TGF‐β signalling
The transforming growth factor (TGF)‐β signalling is involved in diverse cellular processes, such as cell proliferation, differentiation, apoptosis and migration.90, 91 In the tumour development, TGF‐β signalling plays an environment‐dependent role: during the early phases, TGF‐β primarily acts as a tumour suppressor, whereas in the later phases, TGF‐β signalling promotes invasion and metastases of tumour.92, 93, 94 TGF‐β activates cyclin‐dependent kinase inhibitors, p15 and p21, and suppresses CDK2 (cyclin‐dependent kinase 2) and cyclin E, thereby exerting anti‐proliferative effects.95, 96 In breast cancer cell lines, the TGF‐β isoforms, especially TGF‐β2, are downregulated at both mRNA and protein levels when JMJD6 is overexpressed.12 Therefore, JMJD6 may mediate cellular proliferation in part by suppressing TGF‐β2. However, further studies are needed because TGF‐β2 cannot explain the cell cycle arrest in some cell lines.
5.2. JMJD6 interacts with Brd4
Brd4 is a well‐studied member of BET domain family of proteins which are characterized by two conserved N‐terminal bromodomains (BD1 and BD2) and an extraterminal (ET) domain.97 Brd4 binds to acetylated lysine residues on histone tails and other nuclear proteins through bromodomains which have modest affinity for acetylated lysine in a range of polypeptide contexts, and recruits transcriptional regulators such as positive transcription elongation factor b (P‐TEFb) via CTD (carboxyl‐terminal domain) and mediator complex to influence gene expression.97, 98, 99, 100, 101 Cancer‐associated genes seem to be selectively dependent on Brd4, which plays a key role in cancer development.102 In addition to regulating transcription, Brd4 also affects many processes like DNA damage repair and checkpoint activation or telomere homoeostasis.102
The interaction between ET domain of Brd4 and JMJD6 has been identified using proteomic analysis in the initial studies.103 ET domain recognizes the α6 helix of JMJD6.104 As one of the ET domain interactors, JMJD6 has been shown to be critical for P‐TEFb‐independent transcriptional activation of many target genes of Brd4.103 A subsequent study described more detailed investigations on the interaction between Brd4 and JMJD6, and indicated that the JmjC domains and amino‐terminal of JMJD6 and the ET domain of Brd4 mediate this interaction.11 In particular, in the process of P‐TEFb activation and promoter‐proximal polymerase II (Pol II) pause release of a large numbers of genes, both JMJD6 and Brd4 are essential.11, 105 The pause release function of the JMJD6 and Brd4 is primarily based on their co‐binding to distal enhancers, termed anti‐pause enhancers (A‐PEs).11, 73 In terms of mechanism, JMJD6 demethylates H4R3me2(s) (a repressive histone mark) and the methyl cap of 7SK snRNA (a “reader” for H4R3me2(s)), and causes dismissal of the inhibitory complex 7SK snRNA/HEXIM1, thus inducing the activation of P‐TEFb, and permitting subsequent pause release for transcriptional elongation (Figure 4).11 It is noteworthy that either JMJD6 or Brd4 can function independently in promoter‐proximal pause release for some transcription units.11
Figure 4.
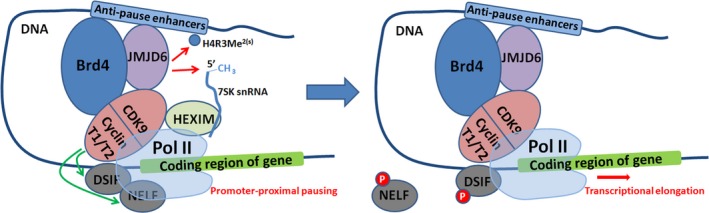
Transcriptional pause release regulation. JMJD6 and Brd4 demethylate H4R3Me2(s) and the methyl cap of 7SK, and cause dismissal of the inhibitory complex 7SK snRNA/HEXIM1, thus inducing the activation of p‐TEFb. P‐TEFb is a heterodimer of cyclin‐dependent kinase 9 (CDK9) and one of cyclin subunit (cyclin T1 or cyclin T2). Activated P‐TEFb phosphorylates RNA pol II (two negative factors, DSIF and NELF, are targeted), permitting subsequent pause release for transcriptional elongation
It has been demonstrated that JMJD6, independently of its catalytic activity, participates in the regulation of DNA damage response signalling in cells by interacting with Brd4.106 JMJD6 is recruited to DNA damage sites and limits the spreading of RNF168‐catalysed histone ubiquitination around DNA double‐strand breaks (DSBs).106 Furthermore, JMJD6 controls the subsequent recruitment of repair proteins, while the expressions of these proteins are not downregulated. Since the spreading of ubiquitination catalysed by RNF168 induces an ataxia telangiectasia‐mutated (ATM)—dependent transcriptional silencing programme in cis to DSBs, JMJD6 plays a role in limiting transcriptional silencing.107 This JMJD6‐mediated DNA damage response is Brd4‐dependent because Brd4 is necessary for the recruitment of JMJD6.106
5.3. JMJD6 regulates ERα‐dependent enhancer and coding gene activation
Oestrogen is a member of steroid hormone family and sustained exposure to oestrogen increases the risk of breast cancer and promotes cancer progression by stimulating proliferation of cancer cells.108, 109 The effects of oestrogen on normal and malignant breast tissues are mainly mediated by ERα, and about 70% of breast cancers are ERα positive.110 JMJD6 has been reported to demethylate the ERa on R260 to regulate the function of ERa.45 JMJD6 recruitment is required for RNA Pol II recruitment and enhancer RNA production of ERα‐bound active enhancers, leading to transcriptional pause release of cognate oestrogen target genes.111 Mediator complex subunit 12 (MED12) is involved in transcriptional regulation of a variety of signalling pathways, including oestrogen‐induced transcriptional activation.112 Mechanistically, JMJD6 specifically interacts with C‐terminus of MED12 and regulates its recruitment to ERα‐bound active enhancers, thereby affecting oestrogen‐induced transcriptional activation.111 Furthermore, JMJD6 is required for MED12 interaction with CARM1 (co‐activator associated arginine methyltransferase 1).111 CARM1 methylates the C‐terminus of MED12, which is necessary for MED12 binding with chromatin and transcriptional regulation.113 In mice model of breast cancer, JMJD6 knock‐down reduces the effects of oestrogen‐induced tumorigenesis, and this is dependent on the enzymatic activities of JMJD6.111 Therefore, JMJD6 is a critical regulator of ERα‐dependent enhancer and coding gene activation through modulating the recruitment of MED12.
5.4. JMJD6 suppresses HNF4α expression
A growing number of studies have shown that the expression of hepatocyte nuclear factor 4α (HNF4α) is reduced in cancers of multiple organs that normally express HNF4α.114 HNF4α is a member of the nuclear receptor superfamily and participates in regulating epithelial junctions, cellular metabolism, differentiation and proliferation of liver and intestinal epithelial cells.115 It has been demonstrated that downregulation of HNF4α promotes tumorigenesis in liver and colon, and reexpression of HNF4α represses cancer progression.114 Arginine methylation level in hepatocytes is mainly controlled by the activity of the protein arginine methyl transferase (PRMT) PRMT1 and the demethylase JMJD6.116 PRMT1 directly upregulates the expression of HNF4α through arginine methylation at the HNF4α promoter, whereas JMJD6 demethylates the HNF4α promoter and suppresses its expression.116 In human hepatocellular cancer specimens, a strong association between arginine methylation and HNF4α level has been observed.116 Therefore, in hepatocytes, PRMT1 and JMJD6 reciprocal regulate arginine methylation level and control HNF4α activity, which may be associated with the development of liver cancer.116 In addition, loss of arginine methylation and downregulation of HNF4α expression may contribute to alcohol‐associated liver cancer.116
5.5. JMJD6 upregulates CDK4
Cyclin‐dependent kinase 4 (CDK4) plays a key role in regulating cell cycle G1 phase progression and the G1‐S transition.117 Aberrant CDK4 expression may result in increased proliferation, which is frequently observed in many types of cancer, including breast cancer, hepatocellular cancer and melanoma.118, 119, 120, 121 JMJD6 promotes CDK4 expression by suppressing H4R3me2(s) binding on the CDK4 promoter.17 Furthermore, JMJD6 interacts with p300/CBP‐associated factor (PCAF), a member of the GCN5‐related N‐acetyl transferase (GNAT) family of protein acetyltransferases, to regulate the histone modifications on the CDK4 promoter.17 The evidence suggesting that CDK4 is a necessary downstream effector of JMJD6 in regulating hepatoma cell proliferation also comes from the observations that the ability of JMJD6 to promote cancer cell proliferation can be abolished by inhibiting CDK4.17 Taken together, JMJD6 may promote hepatocellular cancer progression by targeting CDK4.
5.6. JMJD6 regulates angiogenesis
Abnormal splicing variants may contribute to the development of cancer in humans. It was suggested that JMJD6 regulates the splicing of vascular endothelial growth factor receptor 1 (Flt1) and controls angiogenic sprouting. Downregulation of JMJD6 alters the splicing of Flt1 and increases the levels of its soluble form which binds to vascular endothelial growth factors (VEGF) and placental growth factor, thus inhibiting angiogenesis.50, 122, 123, 124 The role of JMJD6 in splicing regulation may be achieved by its interaction with RS domains (domains rich in alternating arginine and serine residues) of serine and arginine‐rich (SR) proteins and SR‐like splicing factors, especially the splicing factor U2AF65 (Figure 5).23 JMJD6 interacts with U2AF65 that binds to Flt1 mRNA.50 More recently, it was reported that JMJD6 and U2AF65 directly bind to pre‐mRNA and coregulate a large set of alternative splicing events.125
Figure 5.
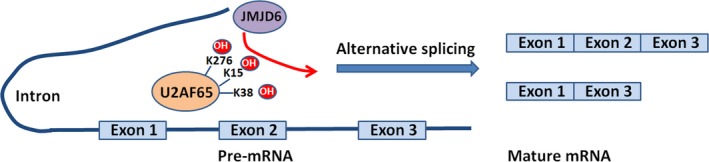
JMJD6 participates in pre‐mRNA splicing regulation through mediating lysine hydroxylation of SR protein and SR‐like splicing factors. For example: JMJD6 hydroxylates the lysine of U2AF65 (K15, 38 and 276), thus regulating alternative splicing
5.7. JMJD6 acts as a tyrosine kinase and promotes autophagy
In a recent study of triple‐negative breast cancer, JMJD6 has been shown to have intrinsic tyrosine kinase activity. JMJD6 phosphorylates Y39 of histone H2A.X (H2A.XY39ph) using ATP and GTP as phosphate donors. It has been reported that phosphorylation of Y39 positively regulates DNA damage response and is related to cancer progression.126 Not surprisingly, phosphorylation of Y39 is increased in various cancer cell lines and is associated with histological grade, tumour size and stage and survival of patients.126 Increased JMJD6 and H2A.XY39ph promote autophagy and triple‐negative breast cancer growth by modulating the expression of autophagy‐related proteins (ATG), including ATG5, ATG7, ATG12 and ATG13.10 Notably, simultaneous blocking tyrosine kinase activity of JMJD6 and autophagy is more effective in reducing the growth of triple‐negative breast cancer in mice than blocking tyrosine kinase activity of JMJD6 or autophagy alone.10 Therefore, the JMJD6‐H2A.XY39ph axis promotes the growth of triple‐negative breast cancer through the autophagy pathway.
5.8. JMJD6 induces IL4 transcription and maintains cancer stemness properties
Interleukin‐4 (IL‐4) is a multifunctional cytokine that can facilitate tumour growth and metastasis.127 In various types of cancer, IL‐4 is overexpressed, including colon cancer, pancreatic cancer and prostate cancer.127, 128, 129 In a study of oral squamous cell carcinoma, JMJD6 was found to be associated with cancer stem cell phenotype.66 In oral squamous cell carcinoma cell lines (SCC9/TNF and UM17b), knock‐down of JMJD6 suppresses IL‐4 mRNA expression.66 Subsequently, chromatin immunoprecipitation assay suggested that JMJD6 binds to the promoter of IL4.66 Furthermore, the addition of recombinant human IL‐4 to the JMJD6‐knock‐down oral squamous cell carcinoma cells rescues the stem‐like properties of cancer stem cells, while IL4 neutralizing suppresses the stem‐like properties.66 Therefore, JMJD6 induces IL4 transcription by binding to its promoter and acts as a regulator of oral cancer stem cell phenotype.
5.9. JMJD6 alters HOTAIR expression
The long non‐coding RNA HOX transcript antisense intergenic RNA (HOTAIR) is a key regulator of chromatin dynamics and is dysregulated in a variety of cancers.130, 131, 132 Overexpression of HOTAIR in epithelial cancer cells induces genome‐wide retargeting of polycomb repressive complex 2 (PRC2) and leads to altered histone methylation and gene expression profiles, resulting in tumour initiation and progression.130 JMJD6 may alter HOTAIR expression in a tumour‐specific manner.133 JMJD6 physically binds upstream of the HOTAIR transcription start site (−123 to −103 bp), and this process is independent of enzymatic activity of JMJD6.133 Inhibition of JMJD6 activity may reduce HOTAIR level, thus reducing tumour growth and improving the prognosis of breast cancer patients.
5.10. HOXB9 targets JMJD6
As a member of homeobox superfamily, homeobox‐containing B9 (HOXB9) has been demonstrated to function in embryonic development and human cancer progression.134, 135 In contrast to the wild‐type HOXB9, HOXB9 acetylated at lysine 27 decreases its ability to promote the migration and growth of lung cancer cells in mice through direct occupying the promoter of its target gene JMJD6 and suppressing the transcription of JMJD6.72
6. CONCLUSIONS
The discovery of JMJD6 as arginine demethylase, lysyl hydroxylase and tyrosine kinase of histone suggests that the protein plays a role in chromatin configuration and epigenetic regulation. Loss‐of‐function studies in knockout mice have shown that JMJD6 plays an important role in embryogenesis and tissue differentiation. In addition to gene mutations, epigenetic modifications can also disrupt gene expression and cause malignant cell transformation. Not surprisingly, JMJD6 has been demonstrated to be upregulated in a wide spectrum of human cancers, and the enzymatic activities of JMJD6 have been shown to be related to its role in cancer. Although precise mechanisms by which JMJD6 promotes tumorigenesis and tumour progression have not been elucidated, it has been well established that interaction of JMJD6 with other cancer‐related signalling pathways is one of the underlying mechanisms. Furthermore, it has been demonstrated that JMJD6 is involved in resistance to chemotherapy, such as doxorubicin, methotrexate and etoposide. By virtue of its important role in cancer, JMJD6 stands as an attractive therapeutic target. We hypothesize that inhibition of JMJD6 as a monotherapy or in combination with other anti‐tumour drugs may produce good anti‐tumour effects in human cancer.
Overall, considering the role of JMJD6 in cancer progression, we believe that targeting JMJD6 is a potential strategy for developing novel therapeutics for cancer management. However, a large number of preclinical and clinical experiments are needed to verify the effectiveness of JMJD6 inhibition in cancer therapy. Continued efforts to elucidate the physiological functions of JMJD6 and the mechanisms by which JMJD6 promotes cancer progression are also critical.
CONFLICT OF INTEREST
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
JY and SC curated the data and involved in formal analysis and writing—original draft. YY involved in formal analysis and writing—review and editing. XM wrote—original draft and wrote—review and editing. YY and BS wrote—review and editing. SY wrote—review and editing and involved in figure drawing. YW conceptualized and supervised the study. XW conceptualized, supervised, validated and wrote—review editing. All authors read and approved the final manuscript.
Yang J, Chen S, Yang Y, et al. Jumonji domain‐containing protein 6 protein and its role in cancer. Cell Prolif. 2020;53:e12747 10.1111/cpr.12747
Yang, Chen and Ma contributed equally to this work.
Funding information
This work is supported by the National Natural Science Foundation of China (No. 81602492), the National Key Research and Development Program of China (No. 2016YFA0201402), the National Major Scientific, Technological Special Project for “Significant New Drugs Development” (No. 2018ZX09733001) and by the Excellent Youth Foundation of Sichuan Scientific Committee Grant in China (No. 2019JDJQ0008).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12‐27. [DOI] [PubMed] [Google Scholar]
- 2. Kanwal R, Gupta K, Gupta S. Cancer epigenetics: an introduction. Methods Mol Biol. 2015;1238:3‐25. [DOI] [PubMed] [Google Scholar]
- 3. Kanwal R, Gupta S. Epigenetics and cancer. J Appl Physiol. 2010;109:598‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31:27‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klose RJ, Zhang Y. Regulation of histone methylation by demethylimination and demethylation. Nat Rev Mol Cell Biol. 2007;8:307‐318. [DOI] [PubMed] [Google Scholar]
- 6. Pedersen MT, Helin K. Histone demethylases in development and disease. Trends Cell Biol. 2010;20:662‐671. [DOI] [PubMed] [Google Scholar]
- 7. Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine‐specific clearance of apoptotic cells. Nature. 2000;405:85‐90. [DOI] [PubMed] [Google Scholar]
- 8. Chang B, Chen Y, Zhao Y, Bruick RK. JMJD6 is a histone arginine demethylase. Science. 2007;318:444‐447. [DOI] [PubMed] [Google Scholar]
- 9. Bose J, Gruber AD, Helming L, et al. The phosphatidylserine receptor has essential functions during embryogenesis but not in apoptotic cell removal. J Biol. 2004;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu Y, Long YH, Wang SQ, et al. JMJD6 regulates histone H2A.X phosphorylation and promotes autophagy in triple‐negative breast cancer cells via a novel tyrosine kinase activity. Oncogene. 2019;38:980‐997. [DOI] [PubMed] [Google Scholar]
- 11. Liu W, Ma Q, Wong K, et al. Brd4 and JMJD6‐associated anti‐pause enhancers in regulation of transcriptional pause release. Cell. 2013;155:1581‐1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee YF, Miller LD, Chan XB, et al. JMJD6 is a driver of cellular proliferation and motility and a marker of poor prognosis in breast cancer. Breast Cancer Res. 2012;14:R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou DX, Zhou D, Zhan SQ, et al. Inhibition of JMJD6 expression reduces the proliferation, migration and invasion of neuroglioma stem cells. Neoplasma. 2017;64:700‐708. [DOI] [PubMed] [Google Scholar]
- 14. Poulard C, Rambaud J, Lavergne E, et al. Role of JMJD6 in breast tumourigenesis. PLoS ONE. 2015;10:e0126181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang J, Ni SS, Zhao WL, Dong XC, Wang JL. High expression of JMJD6 predicts unfavorable survival in lung adenocarcinoma. Tumor Biol. 2013;34:2397‐2401. [DOI] [PubMed] [Google Scholar]
- 16. Wang F, He L, Huangyang P, et al. JMJD6 promotes colon carcinogenesis through negative regulation of p53 by hydroxylation. PLoS Biol. 2014;12:e1001819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wan J, Liu H, Yang L, Ma L, Liu J, Ming L. JMJD6 promotes hepatocellular carcinoma carcinogenesis by targeting CDK4. Int J Cancer. 2018;144:2489‐2500. [DOI] [PubMed] [Google Scholar]
- 18. Fadok VA, de Cathelineau A, Daleke DL, Henson PM, Bratton DL. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J Biol Chem. 2001;276:1071‐1077. [DOI] [PubMed] [Google Scholar]
- 19. Wang X, Wu YC, Fadok VA, et al. Cell corpse engulfment mediated by C. elegans phosphatidylserine receptor through CED‐5 and CED‐12. Science. 2003;302:1563‐1566. [DOI] [PubMed] [Google Scholar]
- 20. Hong JR, Lin GH, Lin CJ, et al. Phosphatidylserine receptor is required for the engulfment of dead apoptotic cells and for normal embryonic development in zebrafish. Development. 2004;131:5417‐5427. [DOI] [PubMed] [Google Scholar]
- 21. Cui P, Qin B, Liu N, Pan G, Pei D. Nuclear localization of the phosphatidylserine receptor protein via multiple nuclear localization signals. Exp Cell Res. 2004;293:154‐163. [DOI] [PubMed] [Google Scholar]
- 22. Cikala M, Alexandrova O, David CN, et al. The phosphatidylserine receptor from Hydra is a nuclear protein with potential Fe(II) dependent oxygenase activity. BMC Cell Biol. 2004;5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Webby CJ, Wolf A, Gromak N, et al. Jmjd6 catalyses lysyl‐hydroxylation of U2AF65, a protein associated with RNA splicing. Science. 2009;325:90‐93. [DOI] [PubMed] [Google Scholar]
- 24. Kwok J, O'Shea M, Hume DA, Lengeling A. Jmjd6, a JmjC dioxygenase with many interaction partners and pleiotropic functions. Front Genet. 2017;8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miotti S, Gulino A, Ferri R, et al. Antibody‐mediated blockade of JMJD6 interaction with collagen I exerts antifibrotic and antimetastatic activities. FASEB J. 2017;31:5356‐5370. [DOI] [PubMed] [Google Scholar]
- 26. Hahn P, Bose J, Edler S, Lengeling A. Genomic structure and expression of Jmjd6 and evolutionary analysis in the context of related JmjC domain containing proteins. BMC Genom. 2008;9:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clissold PM, Ponting CP. JmjC: cupin metalloenzyme‐like domains in jumonji, hairless and phospholipase A2beta. Trends Biochem Sci. 2001;26:7‐9. [DOI] [PubMed] [Google Scholar]
- 28. Aik W, McDonough MA, Thalhammer A, Chowdhury R, Schofield CJ. Role of the jelly‐roll fold in substrate binding by 2‐oxoglutarate oxygenases. Curr Opin Struct Biol. 2012;22:691‐700. [DOI] [PubMed] [Google Scholar]
- 29. Clifton IJ, McDonough MA, Ehrismann D, Kershaw NJ, Granatino N, Schofield CJ. Structural studies on 2‐oxoglutarate oxygenases and related double‐stranded beta‐helix fold proteins. J Inorg Biochem. 2006;100:644‐669. [DOI] [PubMed] [Google Scholar]
- 30. McDonough MA, Loenarz C, Chowdhury R, Clifton IJ, Schofield CJ. Structural studies on human 2‐oxoglutarate dependent oxygenases. Curr Opin Struct Biol. 2010;20:659‐672. [DOI] [PubMed] [Google Scholar]
- 31. Bottger A, Islam MS, Chowdhury R, Schofield CJ, Wolf A. The oxygenase Jmjd6—a case study in conflicting assignments. Biochem J. 2015;468:191‐202. [DOI] [PubMed] [Google Scholar]
- 32. Wolf A, Mantri M, Heim A, et al. The polyserine domain of the lysyl‐5 hydroxylase Jmjd6 mediates subnuclear localization. Biochem J. 2013;453:357‐370. [DOI] [PubMed] [Google Scholar]
- 33. Hong X, Zang J, White J, et al. Interaction of JMJD6 with single‐stranded RNA. Proc Natl Acad Sci U S A. 2010;107:14568‐14572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reeves R, Nissen MS. The A.T‐DNA‐binding domain of mammalian high mobility group I chromosomal proteins. A novel peptide motif for recognizing DNA structure. J Biol Chem. 1990;265:8573‐8582. [PubMed] [Google Scholar]
- 35. Huth JR, Bewley CA, Nissen MS, et al. The solution structure of an HMG‐I(Y)‐DNA complex defines a new architectural minor groove binding motif. Nat Struct Biol. 1997;4:657‐665. [DOI] [PubMed] [Google Scholar]
- 36. Howard MB, Ekborg NA, Taylor LE, Hutcheson SW, Weiner RM. Identification and analysis of polyserine linker domains in prokaryotic proteins with emphasis on the marine bacterium Microbulbifer degradans. Protein Sci. 2004;13:1422‐1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vangimalla SS, Ganesan M, Kharbanda KK, Osna NA. Bifunctional enzyme JMJD6 contributes to multiple disease pathogenesis: new twist on the old story. Biomolecules. 2017;7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tibrewal N, Liu T, Li H, Birge RB. Characterization of the biochemical and biophysical properties of the phosphatidylserine receptor (PS‐R) gene product. Mol Cell Biochem. 2007;304:119‐125. [DOI] [PubMed] [Google Scholar]
- 39. Han G, Li J, Wang Y, et al. The hydroxylation activity of Jmjd6 is required for its homo‐oligomerization. J Cell Biochem. 2012;113:1663‐1670. [DOI] [PubMed] [Google Scholar]
- 40. Hahn P, Wegener I, Burrells A, et al. Analysis of Jmjd6 cellular localization and testing for its involvement in histone demethylation. PLoS ONE. 2010;5:e13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang Y, Reinberg D. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev. 2001;15:2343‐2360. [DOI] [PubMed] [Google Scholar]
- 42. Wysocka J, Allis CD, Coonrod S. Histone arginine methylation and its dynamic regulation. Front Biosci. 2006;11:344‐355. [DOI] [PubMed] [Google Scholar]
- 43. Lawrence P, Conderino JS, Rieder E. Redistribution of demethylated RNA helicase A during foot‐and‐mouth disease virus infection: role of Jumonji C‐domain containing protein 6 in RHA demethylation. Virology. 2014;452‐453:1‐11. [DOI] [PubMed] [Google Scholar]
- 44. Gao WW, Xiao RQ, Peng BL, et al. Arginine methylation of HSP70 regulates retinoid acid‐mediated RARbeta2 gene activation. Proc Natl Acad Sci U S A. 2015;112:E3327‐E3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Poulard C, Rambaud J, Hussein N, Corbo L, Le Romancer M. JMJD6 regulates ERalpha methylation on arginine. PLoS ONE. 2014;9:e87982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tikhanovich I, Kuravi S, Artigues A, et al. Dynamic arginine methylation of tumor necrosis factor (TNF) receptor‐associated factor 6 regulates toll‐like receptor signaling. J Biol Chem. 2015;290:22236‐22249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu TF, Yao YL, Lai IL, Lai CC, Lin PL, Yang WM. Loading of PAX3 to mitotic chromosomes is mediated by arginine methylation and associated with Waardenburg syndrome. J Biol Chem. 2015;290:20556‐20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chowdhury R, Yeoh KK, Tian YM, et al. The oncometabolite 2‐hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 2011;12:463‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mantri M, Krojer T, Bagg EA, et al. Crystal structure of the 2‐Oxoglutarate‐ and Fe(II)‐dependent lysyl hydroxylase JMJD6. J Mol Biol. 2010;401:211‐222. [PubMed] [Google Scholar]
- 50. Boeckel JN, Guarani V, Koyanagi M, et al. Jumonji domain‐containing protein 6 (Jmjd6) is required for angiogenic sprouting and regulates splicing of VEGF‐receptor 1. Proc Natl Acad Sci U S A. 2011;108:3276‐3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Unoki M, Masuda A, Dohmae N, et al. Lysyl 5‐hydroxylation, a novel histone modification, by jumonji domain containing 6 (JMJD6). J Biol Chem. 2013;288:6053‐6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mantri M, Loik ND, Hamed RB, Claridge TDW, McCullagh JSO, Schofield CJ. The 2‐oxoglutarate‐dependent oxygenase JMJD6 catalyses oxidation of lysine residues to give 5S‐hydroxylysine residues. ChemBioChem. 2011;12:531‐534. [DOI] [PubMed] [Google Scholar]
- 53. Ebersole JL. Hypoxia‐inducible transcription factors, HIF1A and HIF2A, increase in aging mucosal tissues. Plant Physiol. 2018;154:452‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343‐354. [DOI] [PubMed] [Google Scholar]
- 55. Li L, Wang Q, Yuan Z, et al. LncRNA‐MALAT1 promotes CPC proliferation and migration in hypoxia by up‐regulation of JMJD6 via sponging miR‐125. Biochem Biophys Res Commun. 2018;499:711‐718. [DOI] [PubMed] [Google Scholar]
- 56. Rose NR, McDonough MA, King ON, Kawamura A, Schofield CJ. Inhibition of 2‐oxoglutarate dependent oxygenases. Chem Soc Rev. 2011;40:4364‐4397. [DOI] [PubMed] [Google Scholar]
- 57. Wen C, Xu M, Mo C, Cheng Z, Guo Q, Zhu X. JMJD6 exerts function in neuropathic pain by regulating NFkappaB following peripheral nerve injury in rats. Int J Mol Med. 2018;42:633‐642. [DOI] [PubMed] [Google Scholar]
- 58. Lawrence P, Rieder E. Insights into Jumonji C‐domain containing protein 6 (JMJD6): a multifactorial role in foot‐and‐mouth disease virus replication in cells. Virus Genes. 2017;53:340‐351. [DOI] [PubMed] [Google Scholar]
- 59. Lawrence P, Rai D, Conderino JS, Uddowla S, Rieder E. Role of Jumonji C‐domain containing protein 6 (JMJD6) in infectivity of foot‐and‐mouth disease virus. Virology. 2016;492:38‐52. [DOI] [PubMed] [Google Scholar]
- 60. Alahari S, Caniggia I. Differential regulation of jumonji domain containing protein 6 (JMJD6) in gestational diabetes mellitus and preeclampsia. Placenta. 2014;35(9):A88. [Google Scholar]
- 61. Ganesan M, Tikhanovich I, Vangimalla SS, et al. Demethylase JMJD6 as a new regulator of interferon signaling: effects of HCV and ethanol metabolism. Cell Mol Gastroenterol Hepatol. 2018;5:101‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Aprelikova O, Chen K, El Touny LH, et al. The epigenetic modifier JMJD6 is amplified in mammary tumors and cooperates with c‐Myc to enhance cellular transformation, tumor progression, and metastasis. Clin Epigenetics. 2016;8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu X, Si W, Liu X, et al. JMJD6 promotes melanoma carcinogenesis through regulation of the alternative splicing of PAK1, a key MAPK signaling component. Mol Cancer. 2017;16:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Anelli V, Ordas A, Kneitz S, et al. Ras‐induced miR‐146a and 193a target Jmjd6 to regulate melanoma progression. Front Genet. 2018;9:675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105‐111. [DOI] [PubMed] [Google Scholar]
- 66. Lee CR, Lee SH, Rigas NK, et al. Elevated expression of JMJD6 is associated with oral carcinogenesis and maintains cancer stemness properties. Carcinogenesis. 2016;37:119‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dou J, Pan M, Wen P, et al. Isolation and identification of cancer stem‐like cells from murine melanoma cell lines. Cell Mol Immunol. 2007;4:467‐472. [PubMed] [Google Scholar]
- 68. Han X, Du F, Jiang L, et al. A2780 human ovarian cancer cells with acquired paclitaxel resistance display cancer stem cell properties. Oncol Lett. 2013;6:1295‐1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lee SH, Hong HS, Liu ZX, et al. TNFalpha enhances cancer stem cell‐like phenotype via Notch‐Hes1 activation in oral squamous cell carcinoma cells. Biochem Biophys Res Commun. 2012;424:58‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Beck B, Blanpain C. Unravelling cancer stem cell potential. Nat Rev Cancer. 2013;13:727‐738. [DOI] [PubMed] [Google Scholar]
- 71. Zhang J, Ni SS, Zhao WL, Dong XC, Wang JL. High expression of JMJD6 predicts unfavorable survival in lung adenocarcinoma. Tumour Biol. 2013;34:2397‐2401. [DOI] [PubMed] [Google Scholar]
- 72. Wan J, Xu W, Zhan J, et al. PCAF‐mediated acetylation of transcriptional factor HOXB9 suppresses lung adenocarcinoma progression by targeting oncogenic protein JMJD6. Nucleic Acids Res. 2016;44:10662‐10675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Miller TE, Liau BB, Wallace LC, et al. Transcription elongation factors represent in vivo cancer dependencies in glioblastoma. Nature. 2017;547:355‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Northcott PA. Cancer: keeping it real to kill glioblastoma. Nature. 2017;547:291‐292. [DOI] [PubMed] [Google Scholar]
- 75. Zheng H, Tie Y, Fang Z. Jumonji domain‐containing 6 (JMJD6) identified as a potential therapeutic target in ovarian cancer. Signal Transduct Target Ther. 2019;4:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Muller PA, Vousden KH. Mutant p53 in cancer: new functions and therapeutic opportunities. Cancer Cell. 2014;25:304‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nusse R, Clevers H. Wnt/beta‐catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985‐999. [DOI] [PubMed] [Google Scholar]
- 78. Clevers H, van de Wetering M. TCF/LEF factor earn their wings. Trends Genet. 1997;13:485‐489. [DOI] [PubMed] [Google Scholar]
- 79. Zhang X, Gao Y, Lu L, et al. JmjC domain‐containing protein 6 (Jmjd6) derepresses the transcriptional repressor transcription factor 7‐like 1 (Tcf7l1) and is required for body axis patterning during xenopus embryogenesis. J Biol Chem. 2015;290:20273‐20283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhang Z, Yang Y, Zhang X. MiR‐770 inhibits tumorigenesis and EMT by targeting JMJD6 and regulating WNT/beta‐catenin pathway in non‐small cell lung cancer. Life Sci. 2017;188:163‐171. [DOI] [PubMed] [Google Scholar]
- 81. De Luca A, Maiello MR, D'Alessio A, Pergameno M, Normanno N. The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin Ther Targets. 2012;16(Suppl 2):S17‐27. [DOI] [PubMed] [Google Scholar]
- 82. Yang SH, Sharrocks AD, Whitmarsh AJ. MAP kinase signalling cascades and transcriptional regulation. Gene. 2013;513:1‐13. [DOI] [PubMed] [Google Scholar]
- 83. Burotto M, Chiou VL, Lee JM, Kohn EC. The MAPK pathway across different malignancies: a new perspective. Cancer. 2014;120:3446‐3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Slack‐Davis JK, Eblen ST, Zecevic M, et al. PAK1 phosphorylation of MEK1 regulates fibronectin‐stimulated MAPK activation. J Cell Biol. 2003;162:281‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Shrestha Y, Schafer EJ, Boehm JS, et al. PAK1 is a breast cancer oncogene that coordinately activates MAPK and MET signaling. Oncogene. 2012;31:3397‐3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dang CV. MYC on the path to cancer. Cell. 2012;149:22‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8:976‐990. [DOI] [PubMed] [Google Scholar]
- 88. Zindy F, Eischen CM, Randle DH, et al. Myc signaling via the ARF tumor suppressor regulates p53‐dependent apoptosis and immortalization. Genes Dev. 1998;12:2424‐2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Qi Y, Gregory MA, Li Z, Brousal JP, West K, Hann SR. p19ARF directly and differentially controls the functions of c‐Myc independently of p53. Nature. 2004;431:712‐717. [DOI] [PubMed] [Google Scholar]
- 90. Xie F, Ling L, van Dam H, Zhou F, Zhang L. TGF‐beta signaling in cancer metastasis. Acta Biochim Biophys Sin (Shanghai). 2018;50:121‐132. [DOI] [PubMed] [Google Scholar]
- 91. Yang L, Moses HL. Transforming growth factor beta: tumor suppressor or promoter? Are host immune cells the answer? Cancer Res. 2008;68:9107‐9111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13:616‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Heldin CH, Vanlandewijck M, Moustakas A. Regulation of EMT by TGFbeta in cancer. FEBS Lett. 2012;586:1959‐1970. [DOI] [PubMed] [Google Scholar]
- 94. Xu J, Lamouille S, Derynck R. TGF‐beta‐induced epithelial to mesenchymal transition. Cell Res. 2009;19:156‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Robson CN, Gnanapragasam V, Byrne RL, Collins AT, Neal DE. Transforming growth factor‐beta1 up‐regulates p15, p21 and p27 and blocks cell cycling in G1 in human prostate epithelium. J Endocrinol. 1999;160:257‐266. [DOI] [PubMed] [Google Scholar]
- 96. Moustakas A, Pardali K, Gaal A, Heldin CH. Mechanisms of TGF‐beta signaling in regulation of cell growth and differentiation. Immunol Lett. 2002;82:85‐91. [DOI] [PubMed] [Google Scholar]
- 97. Shi J, Vakoc CR. The mechanisms behind the therapeutic activity of BET bromodomain inhibition. Mol Cell. 2014;54:728‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Dey A, Chitsaz F, Abbasi A, Misteli T, Ozato K. The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proc Natl Acad Sci U S A. 2003;100:8758‐8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Filippakopoulos P, Picaud S, Mangos M, et al. Histone recognition and large‐scale structural analysis of the human bromodomain family. Cell. 2012;149:214‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Jang MK, Mochizuki K, Zhou M, Jeong HS, Brady JN, Ozato K. The bromodomain protein Brd4 is a positive regulatory component of P‐TEFb and stimulates RNA polymerase II‐dependent transcription. Mol Cell. 2005;19:523‐534. [DOI] [PubMed] [Google Scholar]
- 101. Yang Z, Yik JH, Chen R, et al. Recruitment of P‐TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol Cell. 2005;19:535‐545. [DOI] [PubMed] [Google Scholar]
- 102. Donati B, Lorenzini E, Ciarrocchi A. BRD4 and cancer: going beyond transcriptional regulation. Mol Cancer. 2018;17:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rahman S, Sowa ME, Ottinger M, et al. The Brd4 extraterminal domain confers transcription activation independent of pTEFb by recruiting multiple proteins, including NSD3. Mol Cell Biol. 2011;31:2641‐2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Konuma T, Yu D, Zhao C, Ju Y, Sharma R, Ren C. Structural mechanism of the oxygenase JMJD6 recognition by the extraterminal (ET) domain of BRD4. Sci Rep. 2017;7:16272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Reppas NB, Wade JT, Church GM, Struhl K. The transition between transcriptional initiation and elongation in E. coli is highly variable and often rate limiting. Mol Cell. 2006;24:747‐757. [DOI] [PubMed] [Google Scholar]
- 106. Huo D, Chen H, Cheng Y, Song X, Zhang K, Li MJ. JMJD6 modulates DNA damage response through downregulating H4K16ac independently of its enzymatic activity. Cell Death Differ. 2019. 10.1038/s41418-019-0397-3. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Shanbhag NM, Rafalska‐Metcalf IU, Balane‐Bolivar C, Janicki SM, Greenberg RA. ATM‐dependent chromatin changes silence transcription in cis to DNA double‐strand breaks. Cell. 2010;141:970‐981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Colditz GA. Relationship between estrogen levels, use of hormone replacement therapy, and breast cancer. J Natl Cancer Inst. 1998;90:814‐823. [DOI] [PubMed] [Google Scholar]
- 109. Le Romancer M, Poulard C, Cohen P, Sentis S, Renoir JM, Corbo L. Cracking the estrogen receptor's posttranslational code in breast tumors. Endocr Rev. 2011;32:597‐622. [DOI] [PubMed] [Google Scholar]
- 110. Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand‐binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474‐1481. [DOI] [PubMed] [Google Scholar]
- 111. Gao WW, Xiao RQ, Zhang WJ, et al. JMJD6 licenses ERalpha‐dependent enhancer and coding gene activation by modulating the recruitment of the CARM1/MED12 co‐activator complex. Mol Cell. 2018;70:340‐357.e348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Philibert RA, Madan A. Role of MED12 in transcription and human behavior. Pharmacogenomics. 2007;8:909‐916. [DOI] [PubMed] [Google Scholar]
- 113. Wang L, Zhao Z, Meyer MB, et al. CARM1 methylates chromatin remodeling factor BAF155 to enhance tumor progression and metastasis. Cancer Cell. 2014;25:21‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Walesky C, Apte U. Role of hepatocyte nuclear factor 4alpha (HNF4alpha) in cell proliferation and cancer. Gene Expr. 2015;16:101‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Babeu JP, Boudreau F. Hepatocyte nuclear factor 4‐alpha involvement in liver and intestinal inflammatory networks. World J Gastroenterol. 2014;20:22‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zhao J, Adams A, Roberts B, et al. Protein arginine methyl transferase 1‐ and Jumonji C domain‐containing protein 6‐dependent arginine methylation regulate hepatocyte nuclear factor 4 alpha expression and hepatocyte proliferation in mice. Hepatology. 2018;67:1109‐1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. O'Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13:417‐430. [DOI] [PubMed] [Google Scholar]
- 118. Hamilton E, Infante JR. Targeting CDK4/6 in patients with cancer. Cancer Treat Rev. 2016;45:129‐138. [DOI] [PubMed] [Google Scholar]
- 119. Yu Q, Sicinska E, Geng Y, et al. Requirement for CDK4 kinase function in breast cancer. Cancer Cell. 2006;9:23‐32. [DOI] [PubMed] [Google Scholar]
- 120. Mahgoub T, Eustace AJ, Collins DM, Walsh N, O'Donovan N, Crown J. Kinase inhibitor screening identifies CDK4 as a potential therapeutic target for melanoma. Int J Oncol. 2015;47:900‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Rivadeneira DB, Mayhew CN, Thangavel C, et al. Proliferative suppression by CDK4/6 inhibition: complex function of the retinoblastoma pathway in liver tissue and hepatoma cells. Gastroenterology. 2010;138:1920‐1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wu FT, Stefanini MO, Mac Gabhann F, Kontos CD, Annex BH, Popel AS. A systems biology perspective on sVEGFR1: its biological function, pathogenic role and therapeutic use. J Cell Mol Med. 2010;14:528‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms‐like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Olsson AK, Dimberg A, Kreuger J, Claesson‐Welsh L. VEGF receptor signalling – in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359‐371. [DOI] [PubMed] [Google Scholar]
- 125. Yi J, Shen HF, Qiu JS, et al. JMJD6 and U2AF65 co‐regulate alternative splicing in both JMJD6 enzymatic activity dependent and independent manner. Nucleic Acids Res. 2017;45:3503‐3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Liu Y, Long YH, Wang SQ, Li YF, Zhang JH. Phosphorylation of H2A.X(T)(yr39) positively regulates DNA damage response and is linked to cancer progression. FEBS J. 2016;283:4462‐4473. [DOI] [PubMed] [Google Scholar]
- 127. Prokopchuk O, Liu Y, Henne‐Bruns D, Kornmann M. Interleukin‐4 enhances proliferation of human pancreatic cancer cells: evidence for autocrine and paracrine actions. Br J Cancer. 2005;92:921‐928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Roca H, Craig MJ, Ying C, et al. IL‐4 induces proliferation in prostate cancer PC3 cells under nutrient‐depletion stress through the activation of the JNK‐pathway and survivin up‐regulation. J Cell Biochem. 2012;113:1569‐1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Todaro M, Alea MP, Di Stefano AB, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin‐4. Cell Stem Cell. 2007;1:389‐402. [DOI] [PubMed] [Google Scholar]
- 130. Gupta RA, Shah N, Wang KC, et al. Long non‐coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071‐1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kogo R, Shimamura T, Mimori K, et al. Long noncoding RNA HOTAIR regulates polycomb‐dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320‐6326. [DOI] [PubMed] [Google Scholar]
- 132. Yang Z, Zhou L, Wu LM, et al. Overexpression of long non‐coding RNA HOTAIR predicts tumor recurrence in hepatocellular carcinoma patients following liver transplantation. Ann Surg Oncol. 2011;18:1243‐1250. [DOI] [PubMed] [Google Scholar]
- 133. Biswas A, Shettar A, Mukherjee G, Kondaiah P, Desai KV. JMJD6 induces HOTAIR, an oncogenic lincRNA, by physically interacting with its proximal promoter. Biochem J. 2018;475:355‐371. [DOI] [PubMed] [Google Scholar]
- 134. Chen F, Capecchi MR. Paralogous mouse Hox genes, Hoxa9, Hoxb9, and Hoxd9, function together to control development of the mammary gland in response to pregnancy. Proc Natl Acad Sci U S A. 1999;96:541‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Fromental‐Ramain C, Warot X, Lakkaraju S, et al. Specific and redundant functions of the paralogous Hoxa‐9 and Hoxd‐9 genes in forelimb and axial skeleton patterning. Development. 1996;122:461‐472. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


