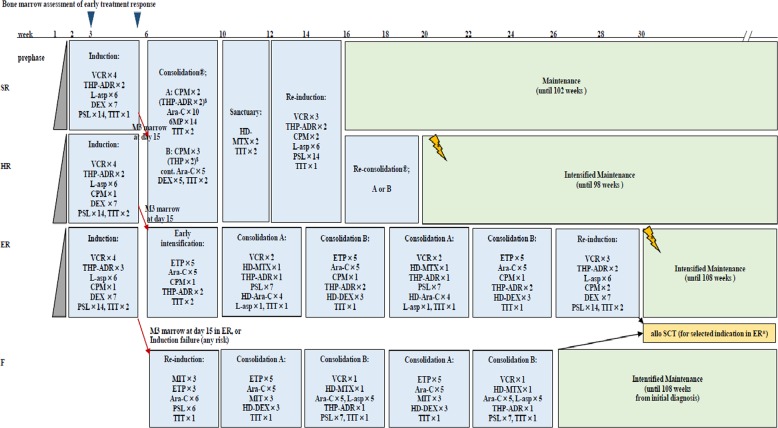
Fig. 1. Outline of JACLS ALL-02 treatment.
Details of treatment elements are listed in Table 1. The therapeutic irradiation dose for patients with initial central nervous system involvement was 12 Gy, irrespective of age. Prophylactic cranial radiotherapy was abolished for non-T cell ALL, irrespective of initial white blood cell count. SR standard risk, HR high risk, ER extremely high risk, PSL prednisone, VCR vincristine, DNR daunorubicin, THP pinorubin, ASP,Escherichia coli l-asparaginase, MTX methotrexate, 6-MP 6-mercaptopurine, ARA-C cytarabine, CPM cyclophosphamide, DEX dexamethasone, DOX doxorubicin, 6-MP 6-mercaptopurine, HD high dose, IT intrathecal, TIT triple intrathecal therapy, G-CSF granulocyte colony-stimulating factor, MT maintenance therapy, SCT stem cell transplantation, pCRT prophylactic cranial radiotherapy. Patients enrolled in JACLS ALL-02 were allocated to three risk groups using the modified NCI criteria, cytogenetics, and treatment responses. Bone marrow examinations were performed on days 15 and 33. Slow early responders, showing M3 marrow (blasts ≥ 25%) on day 15, were shifted to a higher risk group after induction therapy, as augmented post-induction therapy: SR to HR, HR to ER, and ER to F, respectively. Patients who did not achieve complete remission on day 33 were excluded from this study. Patients allocated to ER underwent allogeneic hematopoietic stem cell transplantation if they had an HLA-matched sibling donor.