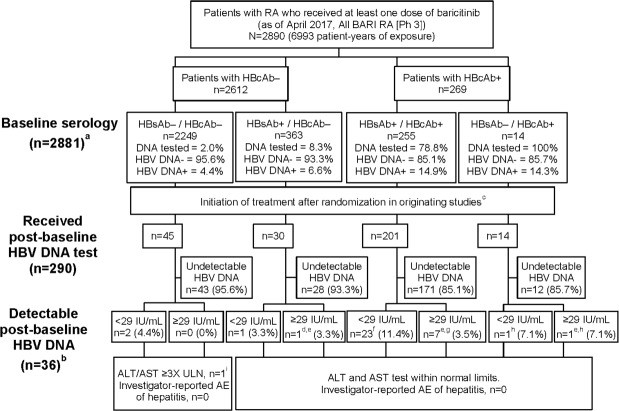
Figure 1.
HBV serology and DNA detectability in patients treated with baricitinib in Phase 3 trials; a total number of patients with baseline serology is n=2881 owing to missing data for nine patients (eight missing surface antibody and one missing core antibody data); b patients with detectable post-baseline HBV DNA were from China (11), Japan (9), Taiwan (6), Israel (2), Greece (2), Russia (2), USA (2), Argentina (1) and South Korea (1); c initial randomised treatment assignment was placebo (concomitant csDMARDs), MTX monotherapy, adalimumab (concomitant MTX), baricitinib 2 mg (concomitant csDMARDs) or 4 mg (monotherapy or concomitant csDMARDs); d one patient (Japan, BARI 4 mg, HBcAb−/HBsAb+/HBV DNA not detected at baseline) had a single post-baseline, detectable HBV DNA result (92, not detected on retest). No antiviral treatment was administered, and there were no hepatic enzyme elevations or other relevant AEs reported; the patient continued baricitinib; e nine patients had quantifiable HBV DNA levels of 31, 36, 60, 76, 92, 256, 257, 869 and 1547 IU/mL; f baseline HBV DNA not detected (17), not tested (5), detectable <29 IU/mL (1); g baseline HBV DNA not detected (5), not tested (2); h baseline HBV DNA not detected; i one patient with ALT/AST ≥3 x ULN was discontinued from study owing to abnormal liver function test. Patient had a single detectable HBV DNA test result <29 IU/mL. At last available observation, ALT and AST were <3 x ULN and <2 x ULN, respectively. AE, adverse event; ALT, alanine transaminases;AST, aspartate transaminases; BARI, baricitinib; csDMARDs, conventional synthetic DMARDs; DMARDs, disease-modifying anti-rheumatic drugs; HBcAb, hepatitis B virus core antibody; HBsAb, hepatitis B virus surface antibody; HBV, hepatitis B virus; MTX, methotrexate; Ph, phase; RA, rheumatoid arthritis;ULN, upper limit of normal.