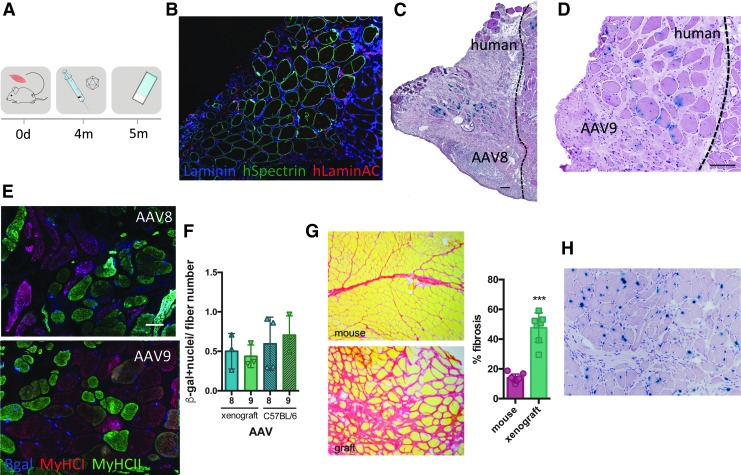
Figure 2.
Intramuscular AAV transduction efficiency in human xenografts. (A) Experimental scheme depicting the xenograft time course. Strips of human biopsies were xenografted into the empty tibial compartment of immunodeficient Rag2−/− Il2rb−/− mice. Four months later, rAAV serotypes 8 and 9 (3 × 1010 vg/TA) were injected into the graft. Mice were sacrificed 4 weeks postinjection and muscles were analyzed. (B) Immunofluorescent staining of human spectrin (green), human lamin a/c (red), and laminin (blue) on a cryosection of human xenograft. (C) Cryosection of xenograft injected with AAV8, stained for β-galactosidase, and counterstained with H&E. Scale bar = 100 μm. (D) Cryosection of xenograft injected with AAV9 and stained for β-galactosidase and colored with H&E. Scale bar = 100 μm. (E) Fiber type (MyHCI-red and MyHCII-green) and β-galactosidase (blue) immunostaining on rAAV8- and rAAV9-injected xenograft. (F) Quantification of cross-sectional xenograft β-galactosidase-positive nuclei related to fiber number on a cross-sectional human xenograft and compared with a C57BL/6 mouse injected with AAV8 and AAV9 (n = 3). ANOVA Tukey test. (G) Sirius red coloration of mouse muscle versus xenograft. Fibrosis was quantified with ImageJ software. ***p < 0.0001, t test. (H) Cryosection of xenograft injected with AAV9, stained for β-galactosidase (blue), and stained with H&E. No β-gal+ interstitial cells are observed. H&E, hematoxylin and eosin.