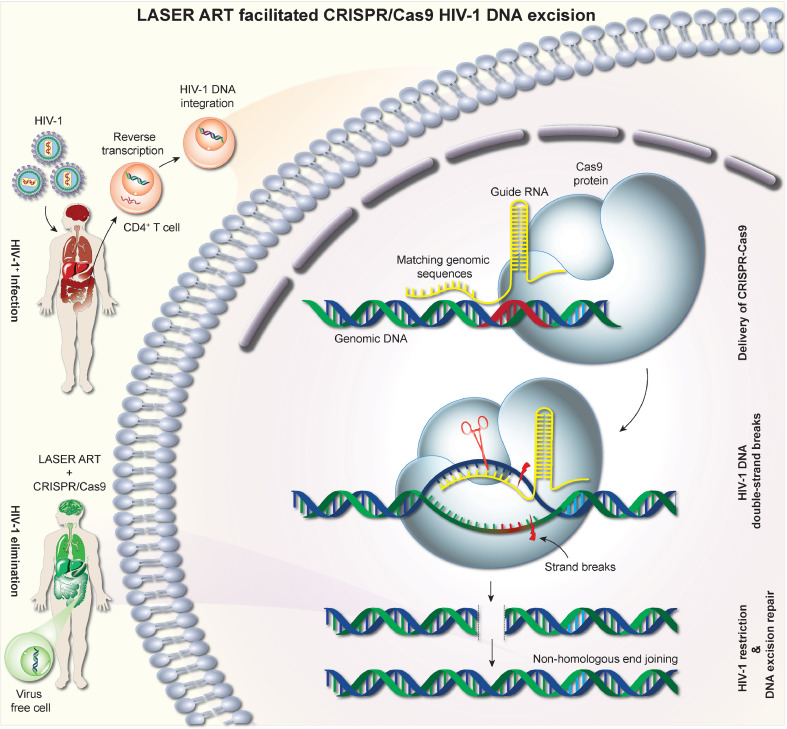
Fig. 4.
Sequential LASER ART and CRISPR-Cas9 for HIV-1 Elimination. Viral entry and fusion require CD4 receptor and CCR5 and CXCR4 coreceptors at the cell surface. Upon entering into virus susceptible CD4+ cells, viral RNA is reverse transcribed into double-stranded circular DNA and integrated into host chromosomal DNA. This provirus can remain quiescent in memory effector T cells. ART drug combinations serve to inhibit virus production at various stages of the HIV life cycle. LASER ART can improve levels of viral restriction by sustaining high drug levels at reservoir sites. Highly specific gRNA based CRISPR-Cas9 targets integrated proviral DNA. The CRISPR-Cas9 utilizes ~100 bp of gRNA to facilitate the Cas9 endonuclease activities at viral integration sites. This made complex then recognizes and cleaves a 20 bp double-stranded DNA target site (known as protospacer DNA) that is complementary to the 5′ end of the gRNA. The double-strand break excises the HIV-1 DNA from the host genome, which gets repaired by non-homologous end-joining to restore the break. This combination of LASER ART and CRISPR-Cas9 has so far achieved viral elimination from a subset of HIV infected animals.