Abstract
The main goals of this research were to use ATR-FTIR spectroscopy associated with multivariate analyses to identify biochemical changes in high and low vigour seed tissues (embryo and endosperm) in response to accelerated ageing and to create a model to predict seed vigour based on spectroscopic data. High-vigour seeds undergo minimal changes in biochemical composition during stress by accelerated ageing while low-vigour seeds are more sensitive to stress and this lower tolerance is associated with reduced lipid and protein content and increased amino acids, carbohydrates and phosphorus compounds in the embryo. High-vigour seeds show an increase in peaks associated with amino acids and phosphorous compounds in the endosperm after 24 h of stress while low-vigour seeds present these high-intensity peaks only after 72 h in the embryo. The results of this research provide the theoretical basis for the genetic improvement of maize cultivars that aim at higher physiological seed quality.
Keywords: Agricultural science, Biochemistry, Natural product chemistry, Food chemistry, ATR-FTIR spectra, Vigour of maize seeds, Physiological quality of seeds, Chemometrics, Functional groups, Biochemical compounds
Agricultural science, Biochemistry, Natural product chemistry, Food chemistry, ATR-FTIR spectra, Vigour of maize seeds, Physiological quality of seeds, Chemometrics, Functional groups, Biochemical compounds.
1. Introduction
Maize (Zea mays L.) seeds carry all the genetic information of the crop and are essential to different purposes, such as for crop production and improvement, agricultural biotechnology, human nutrition, and food security. Seeds can be considered a key element in crop success, although it is dependent on a complex property called vigour (Finch-Savage and Bassel, 2015). Seed quality, defined by improved vigour, is an essential trait, particularly during the current scenario of increasing uncertainty in food production due to climate change and the challenge of population growth expected by 2050 (Hampton et al., 2016). This characteristic, along with other characteristics of seed quality, is a determining factor for the germination and the establishment of crops in a fast and uniform way, in diverse environmental conditions (Rajjou et al., 2012; Marcos Filho, 2015; Finch-Savage and Bassel, 2015; Wen et al., 2018).
The accelerated ageing test is considered one of the most sensitive tests for vigour assessment and consists of keeping the seed under high temperatures and high humidity for a fixed period (Marcos-Filho, 1999; Barreto and Garcia, 2017). Artificial accelerated ageing might cause accumulation of metabolic defects in seeds (Gutierrez et al., 1993) in different proportions than that in natural ageing. This methodology forms the basis of the International Seed Testing Association (ISTA)-validated tests used in commercial seed testing for specific species. Thus, ageing is a key characteristic that is both a cause of differences in vigour and a basis for vigour testing (Finch-Savage and Bassel, 2015). For such claimed reasons the accelerated ageing method was used in this study to model seed vigour and to understand which biochemical changes occur in seed tissues in response to artificial ageing.
The increase in studies for the understanding of vigour has stimulated the investigation of biochemical components and their function in the physiological quality of seeds since they can be used by biotechnology through the manipulation and enrichment of the composition of the tissues (Yan et al., 2014). Seed storage components such as proteins, lipids and carbohydrates, which are the main reserves, are synthesized and stored in the seed tissues during the maturation when they are still in the plant (Coelho and Benedito, 2008; Bewley et al., 2013; Bareke, 2018; Zhao et al., 2018). These storage components are involved in the germination and formation of seedlings providing carbon and nitrogen and, consequently, directly related to vigour (Rajjou et al., 2012; Bewley et al., 2013; Yan et al., 2014; Prazeres and Coelho, 2016a, Prazeres and Coelho, 2016b; Wu et al., 2017; Nerling et al., 2018). Moreover, the research still lacks clarity as to which biochemical component is most affected by deterioration and, consequently, by reduced vigour and where these alterations are occurring in the seed under stress conditions. Our previous work found that seed vigour is related to higher reserve utilization efficiency and mobilization (Andrade et al., 2019).
On the other hand, advances in high-throughput techniques of “omics” sciences have progressively lowered the barrier to accessing omics data (Rajjou et al., 2012; Buescher and Driggers, 2016; Wu et al., 2017). Omics approaches, such as Attenuated Total Reflectance - Fourier Transform Infrared Spectroscopy (ATR-FTIR) generate data to provide biological insight based on statistical inference from datasets that are typically large. Multivariate statistical analysis (PCA, HCA, PLS-DA, amongst others) contribute to reduce the dimensionality of data, to extract important information from the entire spectrum, improving the reliability of the analyses and facilitating the interpretation of the results (Kuhnen et al., 2010). These data may be useful as seed vigour markers and provide information on which biological pathways may be related to the physiological quality (Ventura et al., 2012; Rajjou et al., 2012) and are useful tools for investigating changes in the chemical composition of biological materials in response to stresses (Kumar et al., 2016).
In-depth analyses of the mechanisms involved at the biochemical level have been considered efficient and important tools in many areas of research (Zhang et al., 2012; Oliveira et al., 2016; Uarrota et al., 2018). In seed physiology, there are no reports on the integration of ATR-FTIR to chemometrics to better understand the mechanisms related to seed vigour. In addition, the metabolomic profile of contrasting maize seeds subjected to accelerated ageing conditions remains unknown. In recent years, the mechanisms involved in the manifestation of seed vigour have been extensively studied in different species by many researchers around the world by other methods (Corbineau, 2012; Ventura et al., 2012; Marcos-Filho, 2015; Prazeres and Coelho, 2016a, Prazeres and Coelho, 2016b; Wu et al., 2017; Nerling et al., 2018; Gu et al., 2019). However, the causes that determine this manifestation have not yet been fully elucidated by the research. It is fundamental to understand what determines the expression of seed vigour to improve it and to enhance the establishment of crops, given the great importance of this factor, since it is one of the main factors that are inextricably linked to the success or failure of the future harvest (Marcos-Filho, 2015; Finch-Savage and Bassel, 2015). Besides, it is essential to understand the mechanisms involved in seed deterioration to avoid losses of physiological quality, increasing the longevity of the seeds (Suresh et al., 2019).
In light of the above considerations regarding the contributions of the vigour study to seeds, in this research we tested two hypotheses: (i) there are differences in the biochemical composition of the embryo and endosperm of maize seeds and these differences are related to seed vigour; (ii) the integration of ATR-FTIR profile datasets to chemometric techniques is a powerful tool for modelling biochemical markers related to seed vigour in maize. Based on these hypotheses, the first integrated metabolomic analyses of the embryo and endosperm of two contrasting maize hybrids at vigour level and subjected to accelerated ageing were performed using ATR-FTIR spectroscopy and chemometric analyses (PCA, HCA and PLS-DA). The main objectives of this research were to use spectroscopy associated with multivariate analyses to identify biochemical changes in high and low vigour seeds tissues (embryo and endosperm) in response to accelerated ageing and to create a model for evaluating vigour through techniques based on these changes during stress.
2. Material and methods
2.1. Genotype selection, accelerated ageing test, and extraction of embryo and endosperm
2.1.1. Genotypes selection
Maize genotypes were selected based on the germination rate and vigour. Briefly, eight maize hybrids were subjected to seed physiological analysis (i.e., seed germination rate and vigour by the accelerated ageing test). The germination and vigour were performed as described below.
2.1.2. Germination rate
Eight replications of 50 seeds for each hybrid were used. The methodology previously reported by our research group was used (Andrade et al., 2019) with small modifications. Briefly, seeds were distributed in a germitest paper, moistened with distilled water in the proportion of 2.5 times the weight of dry paper rolls (Brasil, 2009). The rolls were packed in plastic bags and kept in a Mangelsdorf germinator vertically and under 25 ± 2 °C for 7 days. The first count of germination was performed at 4 days and the second count at 7 days. The percentage of germination was calculated as the average number of normal seedlings.
2.1.3. Accelerated ageing test
The methodology previously reported by our research group was also used for the ageing test (Andrade et al., 2019) with small modifications. Briefly, seeds were distributed in a single layer on an aluminium screen, which was placed in boxes of polystyrene crystals (gerbox) containing 40 mL of distilled water (Marcos Filho, 1999). The boxes were closed and placed in an accelerated ageing chamber at 43 °C for 72 h (AOSA, 1983) and at 45 °C for 72 h (de Bittencourt and Vieira, 2006), separately. After this period, the seeds were submitted to the germination test, as previously described. Four replicates of 50 seeds were used for each hybrid and the results were expressed as a percentage of normal seedlings.
2.1.4. Stress condition, extraction of embryo and endosperm
Based on the results of sections 2.1.3 and 2.1.4, two contrasting maize genotypes were selected (one of high vigour -H1 and one of low vigour -H2) and submitted to accelerated ageing stress. Briefly, polystyrene crystal boxes (Gerbox®) (11 cm × 11 cm × 3 cm) with aluminium screens suspended inside were used such that sample seeds were distributed in a single layer on the surface of the screen. 40 mL distilled water was added to the bottom of the boxes to ensure a relative humidity level of the air close to 100% (saturated atmosphere). Boxes were closed and placed in an ageing chamber for 12, 24, 48 and 72 h at 45 °C. Four repetitions were used at each stress period.
2.1.5. Extraction of embryo and endosperm
After each stress period, the samples were removed from the chamber and the seeds were frozen by liquid nitrogen. In addition, samples without stress condition (0 h - control sample) were also frozen. The embryo (embryonic axis and scutellum) separated from the endosperm manually using a scalpel, and immediately frozen in liquid nitrogen, ground using a grinder and stored at -20 °C until the infrared spectroscopy analysis.
2.2. ATR – FTIR spectroscopy
Embryo and endosperm samples from each maize genotype (H1 and H2) at each stress point (0, 12, 24, 48 and 72 h) were evaluated separately. ATR-FTIR spectra (100 mg sample) were recorded in a Bruker IFS-55 (Model Opus v. 5.0, Bruker Biospin, Germany) spectrometer with a DTGS detector equipped with a golden gate single reflection diamond attenuated total reflectance (ATR) accessory, at the transmittance mode from 400 to 4000 cm−1,4 cm−1 of resolution and five replicates of each spectra were collected as described previously by Uarrota et al. (2013), 2014; 2017; 2018, totalling 100 spectra (2 hybrids – high and low vigour; 5 stress times - 0, 12, 24, 48 and 72 h; 2 structures - embryo and endosperm x 5). Spectra were then normalised, baseline corrected and smoothed using Savitzky–Golay derivative function (Savitzky and Golay, 1964). The resolution enhancement (k factor of 1.7, Lorentzian line width of 19 cm−1) was applied using Fourier self deconvolution (Čopíková et al., 2006). The considered region was from 600 to 3200 cm−1. All preprocessing steps were performed in R software (R Core Team, 2019).
2.3. Data mining and statistics
Data of ATR-FTIR spectra were collected, pre-processed as described above and submitted to multivariate statistical analysis (Principal Component Analysis – PCA, Hierarchical Cluster Analysis – HCA and Partial Least Squares Discriminant Analysis – PLS-DA). All analyses were performed in R software (R Core Team, 2019) using scripts produced by the Laboratory of Seed Analysis group.
3. Results and discussion
3.1. Preliminary assay in germination rate and vigour by accelerated ageing
Preliminary results on the physiological quality of the seeds used in the experiment were obtained. The data collected were germination rate and vigour by acceleration ageing test at 45 °C for 72 h (Figure 1). In relation to the germination rate, the observed behaviour between hybrids 1 and 2 was similar (p < 0.05), with percentages of 97 and 98%, respectively. On the other hand, the hybrids presented an extremely contrasting value for initial vigour by accelerated ageing, with significant statistical differences by the Tukey test (p < 0.05), with values of 93% for hybrid 1 and 24% of vigour for the hybrid 2 (Figure 1). In studies related to seed vigour, it is essential that the percentage of germination be similar amongst the materials to be compared, to ensure that the differences are only in vigour and not in the physiological quality as a whole, making comparisons feasible (Sbrussi and Zucarelli, 2014; Marcos-Filho, 2015), based on knowledge that there are genetic diversity for the vigour of maize seeds (Prazeres and Coelho, 2016a, Prazeres and Coelho, 2016b).
Figure 1.
A – Percentages of germination; and B – seed vigour by accelerated ageing for the two hybrids evaluated previously this experiment. Mean values followed by the same lowercase letter belong to the same Tukey test group at 5% probability (p < 0.05).
3.2. ATR-FTIR spectral analyses
Amongst the main objectives of seed vigour testing is the ability to predict and to select seed lots that have the best quality before processing, storing them or taking them to be sown in the field (Marcos-Filho, 2015; Finch-Savage and Bassel, 2015). The basic quality assessment through the germination test and vigour by accelerated ageing is inevitably time-consuming, requiring more than one week to complete and, furthermore, may not always correlate well with emergence under field conditions over a range of environmental conditions. Therefore, it is desirable to have a simple, reliable, accurate, rapid to perform, physiologically informative and relatively inexpensive test of seed quality involving vigour.
In studies of biological materials by ATR-FTIR, there are two important regions for the evaluation of the spectra called fingerprinting region and functional groups region (Li-Chan, 2010; Baker et al., 2014). These regions must be exploited because they generally contain a large number of bands that can overlap each other, causing a single wave number to be related to more than one type of chemical component. The infrared in the middle region (400–4000 cm−1) provides the recognition of functional groups present in chemical compounds (Li-Chan, 2010). It allows the identification of similarities and dissimilarities of the biochemical composition between samples, such as the presence of carbohydrates, proteins and peptides, lipids and fatty acids, nucleic acids, amongst others (Socrates, 2001; Černá et al., 2003; Lopes and Fascio, 2004; Silverstein et al., 2005; Schulz and Baranska, 2007; Kuhnen et al., 2010; López-Sánchez et al., 2010; Kumar et al., 2016). Previous studies of Cozzolino et al. (2013), 2014 have demonstrated the potential of attenuated total refletance combined with multivariate statistics as screening tool to phenotype single seeds of barley, to evaluate fatty acids and total lipids in barley and this technique can be used to better understand the differences between genotypes.
3.2.1. ATR-FTIR spectra of maize embryo of the two hybrids
All peaks detected in the following spectra were identified with the aid of other publications (Socrates, 2001; Černá et al., 2003; Lopes and Fascio, 2004; Silverstein et al., 2005; Schulz and Baranska, 2007; Kuhnen et al., 2010; López-Sánchez et al., 2010; Kumar et al., 2016) because of the high complexity of spectra interpretation and their relation to the functional groups present in biological samples.
Most spectra of the embryo (Table 1) presented high peak intensities in the functional group region (1800-4000 cm−1), with peaks at 2860 and 2930 cm−1 that are related to amino acids, fatty acids, lipids, proteins and peptides (Tables 2A and 2B). Visually by the intensity of the bands, there were no chemical changes in these compounds for the stress times of 0, 12, 24 and 48 h for both hybrids, which means that the behaviour of these compounds over accelerated ageing stress at 45 °C was similar for the high and low vigour hybrids (Table 1).
Table 1.
Chemical groups, representative compounds and spectra wavenumbers found in all ATR-FTIR maize samples of embryos and endosperm of hybrid 1 (high vigour) and hybrid 2 (low-vigour) during the stress time by accelerated ageing.
| Chemical groups | Representatives | FT-IR (cm−1) | References |
|---|---|---|---|
| Proteins | 1655 (1654) | Schulz and Baranska (2007) | |
| Lipids/fatty acids | 1660/1750 (1654/1746) | Schulz and Baranska (2007) | |
| Disaccharides | Sucrose | 1126 (1118) | Schulz and Baranska (2007) |
| Polysaccharides | Cellulose | 1162 (1161) | Schulz and Baranska (2007) |
| Pectin | 1008/1055/1419/1745 (995/1048/1420/1746) | Schulz and Baranska (2007) | |
| Acyclic monoterpenes | Citronellal | 1116 (1118) | Schulz and Baranska (2007) |
| Citronellol | 1377 (1379) | Schulz and Baranska (2007) | |
| Geranyl acetate | 1227/1738 (1238/1746) | Schulz and Baranska (2007) | |
| Monocyclic monoterpenes | Terpinen-4-ol | 1050 (1048) | Schulz and Baranska (2007) |
| Bicyclic monoterpenes | 1,8-Cineol | 1374 (1379) | Schulz and Baranska (2007) |
| α-Pinene | 1658 (1654) | Schulz and Baranska (2007) | |
| Sabinene | 1653 (1654) | Schulz and Baranska (2007) | |
| Sesquiterpenes | α-Bisabolol | 1375 (1379) | Schulz and Baranska (2007) |
| Tetraterpenes | β-Carotene | 1454 (1460) | Schulz and Baranska (2007) |
| Lycopene | 1379 (1379) | Schulz and Baranska (2007) | |
| Akyl halide | C-X | 500-1400 (600/720/995) | Lopes and Fascio (2004) |
| Aril-alkyl-ether | C-O | 1020-1100/1220-1280 | Lopes and Fascio (2004) |
| (1048/1097/1238) | |||
| Aril-alkyl-amine | C-N | 1180-1280/1250-1360 | Lopes and Fascio (2004) |
| (1238/1301/1320) | |||
| Alkyl-ether | C-O | 1080-1150 (1097/1118) | Lopes and Fascio (2004) |
| Alkyl-amine | C-N | 1030–1230 | Lopes and Fascio (2004) |
| (1048/1097/1118/1161/1238) | |||
| Nitro group | NO2 | 1300-1380/1500-1570 | Lopes and Fascio (2004) |
| (1301/1320/1379/1544) | |||
| Alkane | CH3, CH2 | 1375/1465/2840-3000 | Lopes and Fascio (2004) |
| (1379/1460/2856/2925/3009) | |||
| Alckene | C=C | 1620-1680/3000-3100 (1654/3009) | Lopes and Fascio (2004) |
| Aril-ketone | C=O | 1630-1700 (1654) | Lopes and Fascio (2004) |
| Alkyl-ketone | C=O | 1700-1770 (1711/1746) | Lopes and Fascio (2004) |
| Tertiary amide | C=O | 1630-1700 (1654) | Lopes and Fascio (2004) |
| Carboxylic acid | O-H | 2500-3200 (2856/2925/3009) | Lopes and Fascio (2004) |
| Oleophynes | C=C | 680-1000/1630-1680 (995/1654) | Lopes and Fascio (2004) |
| Ester | C=O | 1670-1750 (1711/1746) | Lopes and Fascio (2004) |
| Aromatic | C-H | 3000-3100 (3009) | Lopes and Fascio (2004) |
| Unknown | 1420 | Lopes and Fascio (2004) | |
| Alkynea | C≡C | 2100-2260 (2103/2146) | Lopes and Fascio (2004) |
| Amineb | N-H | 3200-3600 (3200) | Lopes and Fascio (2004) |
| Benzenec | C-H | 690-710/735-770/800-860 (704/763/8611111) | Lopes and Fascio (2004) |
a,b,cChemical groups found in all samples of endosperm during the stress time (0,12, 24, 48 and 72 h) by accelerated ageing independently of seed vigour. bChemical group found in samples of maize embryo of lower vigor (H2_T12 and H2_T72) and cChemical group found in samples of maize embryo of high vigour (H1_T24).
Table 2A.
Specific peaks (wavenumbers) found in each tissue during the stress time in both tissues.
| Hybrid |
Time (hours) |
Wavenumbers (cm−1) |
|---|---|---|
| EMBRYONIC AXIS TISSUE | ||
| H1 | 0 | 720/995/1048/1097/1118/1161/1238/1301/1320/1379/1420/1460/1544/1654/1711/1746/2856/2925/3009 |
| 12 | 669/720/1038/1059/1097/1118/1163/1238/1303/1318/1348/1379/1395/1418/1463/1538/1556/1650/1660/1681/1713/1746/2340/2360/2854/2925/3009 | |
| 24 | 667/683/722/1032/1059/1099/1120/1163/1238/1318/1377/1397/1418/1463/1538/1556/1567/1575/1634/1652/1660/1681/1746/2329/2340/2358/2854/2925/3009 | |
| 48 | 669/1024/1042/1097/1118/1161/1236/1320/1379/1420/1463/1546/1564/1656/1711/1746/2342/2360/2854/2925/3009 | |
| 72 | 669/720/1030/1057/1099/1120/1163/1238/1320/1379/1399/1418/1465/1550/1656/1746/2342/2360/2854/2925/3009 | |
| Similar peaks: 600 | ||
| H2 | 0 | 720/997/1055/1097/1118/1161/1301/1320/1379/1399/1420/1463/1546/1656/1711/1746/2856/2925/3009 |
| 12 | 720/1050/1083/1161/1322/1379/1401/1418/1463/1546/1656/1711/1746/2854/2925/3009/3200 | |
| 24 | 1036/1053/1097/1118/1161/1320/1377/1401/1418/1463/1538/1548/1567/1660/1713/1746/2854/2925/3009 | |
| 48 | 720/1053/1085/1095/1161/1322/1379/1399/1420/1460/1544/1656/1746/2854/2925/3009 | |
| 72 | 1044/1075/1152/1320/1344/1414/1454/1544/1654/2927/3200 | |
| Similar peaks: 600/669/1238/2342/2360 | ||
|
ENDOSPERM TISSUE | ||
| H1 | 0 | 704/861/926/993/1159/1242/1346/1422/1432/1460/1516/1536/1544/1656/1744/2146/2856/2925 |
| 12 | 704/859/926/991/1159/1242/1346/1422/1432/1460/1501/1516/1530/1544/1656/1744/2101/2152/2856/2925 | |
| 24 | 649/669/859/928/993/1157/1242/1346/1416/1434/1442/1452/1463/1503/1514/1530/1548/1564/1644/1658/1724/2089/2113/2127/2144/2927 | |
| 48 | 669/706/861/928/991/1159/1244/1344/1422/1460/1516/1534/1544/1656/1736/2146/2927 | |
| 72 | 706/861/928/993/1159/1244/1424/1460/1518/1536/1658/2103/2146/2927 | |
| Similar peaks: 600/763/1014/1081/1371/2342/2360/3200 | ||
| H2 | 0 | 706/859/926/993/1016/1159/1242/1373/1434/1456/1505/1518/1538/1652/1660/2150/2927 |
| 12 | 704/859/928/995/1016/1159/1242/1346/1373/1422/1432/1460/1542/1656/1742/2152/2927 | |
| 24 | 706/861/928/993/1016/1159/1242/1344/1373/1422/1432/1460/1520/1532/1540/1654/2152/2927 | |
| 48 | 706/861/928/995/1016/1157/1242/1342/1377/1422/1438/1458/1509/1524/1542/1560/1654/1736/2146/2154/2929 | |
| 72 | 706/861/928/993/1014/1159/1244/1344/1371/1424/1432/1460/1542/1656/2101/2144/2152/2929 | |
| Similar peaks: 600/763/1081/3200 | ||
The main and most important differences in the embryo spectra between the hybrids were observed in the region above 2300 cm−1 at 72 h stress. The high-vigour hybrid (H1) did not show changes in the intensity of these bands during the entire stress period, including 72 h, while the low-vigour hybrid (H2) showed an expressive reduction of peak intensity close to 2930 cm−1 (amino acids, fatty acids, lipids, proteins and peptides). In addition, it was observed the absence of peaks close to 2860 cm−1 that is also related to the same compounds and was present in the low-vigour embryo (H2) before, indicating the possible degradation due to stress for 72 h and the lower tolerance to this condition demonstrated by the low-vigour hybrid. The behaviour of the spectral peaks was different between embryos of the hybrids for the time of 72 h, because the response to ageing is dependent on the genotype and initial vigour (Oliveira et al., 2013; Nerling et al., 2013; Prazeres and Coelho, 2016a, Prazeres and Coelho, 2016b).
The high temperature (45 °C) and 100% relative humidity used in artificial ageing in the laboratory may cause damages and decrease the compounds related to the wave number near 2930 cm−1 in less vigorous hybrids, which is related to fatty acids, lipids, proteins, peptides and amino acids (Table 1). The decrease in lipid and fatty acids contents are associated with lipid peroxidation and reduction of antioxidant enzymatic activity caused by accelerated ageing stress in oat, macaw palm and wheat seeds (Xia et al., 2015; Barreto and Garcia, 2017; Tian et al., 2019). Lipid peroxidation leads to the formation of free radicals that accelerate the deterioration of cell membranes, proteins and reduction of enzymatic activity, culminating in the reduction of the viability of the seeds (Tian et al., 2019). In addition, natural or artificial accelerated ageing causes reduction in DNA integrity and protein synthesis, and these changes are closely associated to the reduction of germination under these deterioration conditions (Gutiérrez et al., 1993).
Furthermore, the embryo of the low-vigour hybrid showed an increase in the bands near to 2342 and 2360 cm−1, which are related to amino acids and phosphorus compounds. Most phytic acid, which is the way phosphorus is stored in seeds, is concentrated in the embryo (about 95%) and in aleurone layer in the endosperm (about 5%) of maize seeds, and its action as an antioxidant has been studied in this crop (Lin et al., 2005; Doria et al., 2009; Bewley et al., 2013). In relation to the protein, most are found in the embryo (around 20%) while the endosperm content is close to 10% (Carvalho and Nakagawa, 2012). Due to the observed changes in amino and phosphorus peaks after 72 h in embryo samples, our results suggest the hypothesis that the lower vigour genotype undergoes greater degradation/hydrolysis of proteins and phytic acid, providing amino acids and phosphorus in the embryo in response to stress. Future studies will be carried out to investigate these relationships and the possibility of evaluating the content of phosphorus and amino acids after 72 h of stress at 45 °C to separate vigour levels of hybrid maize seeds and the incorporation of these compounds to obtain seeds with higher physiological quality.
Many peaks, although with lower intensities were identified in the fingerprint region (400-1800 cm−1) for both hybrids in embryo samples. Peaks in the region 900-1200 cm−1 are more correlated with the presence of carbohydrates in general (monosaccharides, oligosaccharides, polysaccharides), besides the presence of some amino acids, nucleic acids and phosphorus compounds. The presence of the band at 1260 cm−1 is related to amino acids, carbohydrates, nucleic acids, phosphate group, proteins and peptides (amide III). Other important bands identified in the embryo spectra of the hybrids were at 1650 and 1550 cm−1, which are correlated to amino acids, nucleic acids, proteins and peptides (amide I and II). The last important band identified was at 1750 cm−1, which is related to acetylated glycosides, amino acids, fatty acids, lipids, phospholipids, pectin, cellulose and nucleic acids (Tables 2A and 2B). These peaks were found in all spectra from time 0 (no stress) without major changes until the 48 h of stress period for the two hybrids. The absence of biochemical changes for both genotypes up to 48 h confirms the relationship of the components of the seed with vigour, indicating that both genotypes present tolerance to stress until this period.
The highest changes in intensity and presence of peaks were observed for the embryo samples collected after 72 h of stress by accelerated ageing for the low-vigour hybrid, indicating behaviour similar to the region of functional groups (1800-4000 cm−1) in the region of fingerprinting (400-1800 cm−1). For the high-vigour hybrid (H1), the bands referring to the fingerprinting region remain unchanged throughout the stress (Tables 2A and 2B). For the low-vigour hybrid (H2), there was an expressive increase in the intensity of the peaks near 1044 and 1075 cm−1, related to soluble sugars in general, structural carbohydrates and components with phosphorus in its composition. In this same hybrid, there was also an increase in intensity at the peak location near 1654 cm−1 (amino acids, nucleic acids and proteins), which was not present at the same intensity at other stress times. In addition, the visual analysis showed absence of the 1750 cm−1 peak (acetylated glycosides, amino acids, fatty acids, lipids, phospholipids, pectin, cellulose and nucleic acids) for the embryo of the low-vigour hybrid, indicating the possible degradation of these compounds and less tolerance to stress by the H2 (Tables 2A and 2B).
3.2.2. ATR-FTIR spectra of maize endosperm samples
Contrarily to those previously observed in embryo, which shows higher peak intensity in the region of the functional groups, the spectra of endosperm samples showed most peaks with high intensities in the fingerprint region (Tables 1, 2A and 2B). This behaviour is directly associated with a higher carbohydrate presence of the composition in the maize seed endosperm tissues, which are identified by the presence of bands in the fingerprint region, especially between 900 and 1200 cm−1 (Socrates, 2001; Černá et al., 2003; Lopes and Fascio, 2004; Silverstein et al., 2005; Schulz and Baranska, 2007; Kuhnen et al., 2010; López-Sánchez et al., 2010; Kumar et al., 2016).
Table 2B.
Related chemical compounds identified in the both tissues during the stress by accelerated ageing.
| Wavenumber | Related chemical compound |
|---|---|
| 1024 | Carbohydrates: cellulose, hemicellulose, polysaccharides, starch, sucrose |
| 1030 | Carbohydrates: amylopectin, amylose, cellulose, galactose, hemicellulose, pectic polysaccharides, pyranose compounds, starch, sucrose |
| 1044 | Carbohydrates: amylopectin, amylose, cellulose, fructose, pectic polysaccharides, pyranose compounds, starch, sucrose, xyloglucan |
| 1059, 1057 | Carbohydrates: amylopectin, amylose, arabinose, cellulose, fructose, glucose, hemicellulose, pectic polysaccharides, pyranose compounds, starch, sucrose |
| 1075 | Carbohydrates: cellulose, hemicellulose, pectic polysaccharides, pyranose compounds, ribose, starch, sucrose, xyloglucan |
| Phosphorus compounds | |
| 1099 | Amino acids |
| Carbohydrates: cellulose, galactose, hemicellulose, pectic polysaccharides, pyranose compounds, ribose, starch, sucrose | |
| Nucleic acids | |
| Phosphorus compounds | |
| 1120 | Amino acids |
| Carbohydrates: cellulose, hemicellulose, pectic polysaccharides, pyranose compounds, starch, sucrose | |
| Nucleic acids | |
| 1180 | Amino acids |
| Carbohydrates: cellulose, hemicellulose, pectic polysaccharides, pyranose compounds, starch, sucrose | |
| Nucleic acids | |
| 1260 | Amino acids |
| Carbohydrates: cellulose, hemicellulose, pectic polysaccharides, pyranose compounds | |
| Nucleic acids | |
| Phosphorus compounds | |
| Proteins and Peptides (amide III) | |
| 1379 | Carbohydrates: cellulose, xyloglucan |
| Lipids | |
| Nucleic acids | |
| 1399 | Amino acids |
| Nucleic acids | |
| Lipids | |
| Protein and peptides: polyglicynes | |
| 1550 | Amino acids |
| Nucleic acids | |
| Protein and peptides (amide II): polypeptides | |
| 1650–1654 | Amino acids |
| Nucleic acids | |
| Protein and peptides (amide I): polyglycines and polypeptides | |
| 1746–1750 | Acetylated glycosides |
| Amino acids | |
| Carbohydrates: pectin, cellulose | |
| Fatty acids, lipids, phospholipids | |
| Nucleic acids | |
| 2342–2360 | Amino acids |
| Phosphorus compounds | |
| 2854 | Amino acids |
| Fatty acids, lipids | |
| Proteins and peptides: polyglycines | |
| 2927–2960 | Amino acids |
| Fatty acids, lipids | |
| Proteins and peptides: polyglycines |
Table 2A summarise the results of endosperm spectra for the two hybrids. The main differences between the endosperm spectra of high and low vigour were observed at 2342 and 2360 cm−1, which are related to amino acids and phosphorus compounds. In all endosperm spectra of the hybrid of high vigour these peaks are present, drawing attention to the increase of intensity after 24 h of stress, whereas in the hybrid of low vigour, these peaks are absent in all periods of stress in the endosperm.
Through the visual inspection of the spectra, it was observed that the embryo spectra presented a higher number of peaks during stress when compared to the endosperm spectra, which means that this morphological structure presents the more severe symptoms of accelerated ageing. Similar results were found by Han et al. (2017) in wheat seeds, where the metabolic changes found in the embryo were superior to those of the endosperm.
3.3. Chemometrics analyses (PCA, HCA and PLS-DA) and model buuilding
3.3.1. Principal Component Analysis (PCA) and hierarchical cluster analysis (HCA)
The PCA was applied to all spectra (600-3200 cm−1) and to selected peaks (regions without peaks were removed) in order to better understand the data patterns, to find clusters of samples and spectral peaks by directing such similarities or differences in the data. HCA and seriated heatmaps were also applied as a complementary analysis of PCA to uncover the factors that boosted the seed vigour.
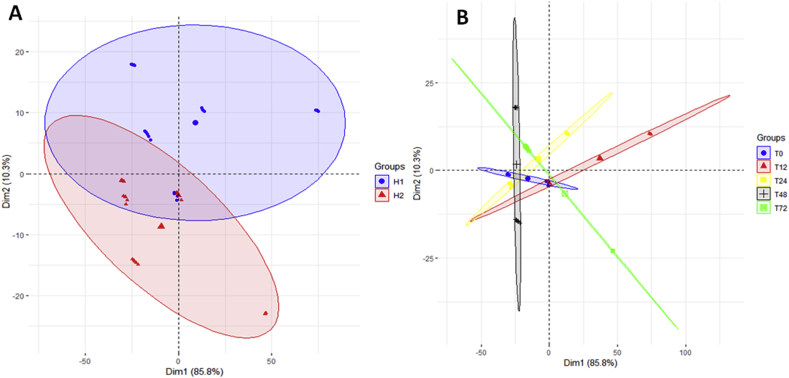
When PCA was applied to the embryo spectra considering the hybrids as a factor, the total variance captured was 96.1%, being 85.8% and 10.3% for PC1 and PC2, respectively (Figure 2A). Although one sample for the H1 hybrid (high vigour) and one sample for the H2 hybrid (low vigour) displaced from the other samples of the same hybrid, there was an effective separation between the two contrasting maize hybrids regarding the level of vigour (Figure 2A). This separation allows us to assume that there are differences between the seed embryos spectra of high and low vigour, regardless of the stress time.
Figure 2.
A – PCA of all spectra region (600-3200 cm−1) of embryo samples taking the two hybrids as a factor. B – PCA of all spectra region of embryo samples taking the stress time as factor.
Taking the stress time as a factor in the same spectra it was not possible to separate the embryo samples meaning that the changes in spectra during the stress time were minimal (Figure 2B). Samples in PC1 (-) were most influenced by fatty acids and lipids while PC1 (+) by polysaccharides. PC2 (-) was associated with proteins and PC2 (+) by polysaccharides, fatty acids and phosphorous compounds.
Regarding the maize endosperm samples (Figure 3A), the total variance captured by PCA taking the maize hybrids as factors was 96%, being 72.4% and 23.6% the variances for PC1 and PC2, respectively. Taking the stress time as a factor, there was a separation between samples, except for the samples of 24 and 48 h of stress of the two hybrids and those stressed during 72 h clustered together independently of the hybrid (Figure 3B). Such results indicate that there are structural changes starting from 24 h of stress. PC1 (+) was correlated to the wavenumber 2300 cm−1, that are related to amino acids and phosphorous compounds, while PC2 (-) was correlated to carbohydrates and proteins.
Figure 3.
A – PCA of all spectra region (600-3200 cm−1) of endosperm samples taking the two hybrids as a factor. B – PCA of all spectra region of endosperm samples taking the stress time as factor.
PCA analysis of the selected peaks of embryo spectra was also done (Figure 4). The total variance captured was 96.5%, being 88.5% and 8% for PC1 and PC2, respectively. Despite the small improvement in the variance, there was no improvement in the grouping of samples when doing the analysis only with the selected peaks when compared to the analysis of the total region of the spectra. Differences were observed only between hybrids (Figure 4A) and not between times of stress (Figure 4B), all others were similar.
Figure 4.
A – PCA of selected peaks of embryo samples taking the two hybrids as a factor. B – PCA of selected peaks of embryo samples taking the stress time as factor.
PCA analysis of the selected peaks of endosperm spectra showed 97.5% of total variance captured (Figure 5A-B), being 86.6% and 10.9% for PC1 and PC2, respectively. Differences were observed between hybrids and time of stress. Samples without stress and 12 h of stress were similar. This tendency was also observed for the stressed samples for 24 and 48 h, while those samples stressed for 72 h were totally different from the others (Figure 5B).
Figure 5.
A – PCA of selected peaks of endosperm samples taking the two hybrids as a factor. B – PCA of selected peaks of endosperm samples taking the stress time as factor.
When hierarchical cluster analysis and seriated heatmaps were applied to the selected spectral peaks for embryo and endosperm, better insights were found. As it can be observed in Figures 6 and 7, in relation to the embryo samples, three different groups can be observed. The first group was composed by H1_T12 (samples of hybrid 1 stressed during 12 h), the second group by H2_T0, H2_T24 and H2_T48 h; and the last group by H1_T0; H1_T24, H1_T48, H1_T72, H2_T12 and H2_T72 (Figure 6A). H2_72 showed to be in the last group, but a deep analysis of seriated heatmaps shows that the H2_72 samples have characteristic peak intensities at 1024, 1059, 1180, 1650 and 900-1099 cm−1 related to polysaccharides, proteins and carbohydrates. The sample H1_12 was separated alone mainly due to the presence of the peaks at 1750 and 2927 cm−1 that are related to acetylated glycosides, amino acids, fatty acids, lipids, phospholipids, proteins and peptides.
Figure 6.
A – HCA of selected peaks of embryo samples; and B – Heatmap of selected peaks of embryo samples.
Figure 7.
A – HCA of selected peaks of endosperm samples; and B – Heatmap of selected peaks of endosperm samples.
HCA (Figure 7A) and seriated heatmaps (Figure 7B) of endosperm samples. The H2_T72 sample was grouped alone in the HCA, but close to the group composed of samples from hybrid 1 (H1_T24 and H1_T72), as it showed the similarity of peaks with these samples (Figure 7A).
The presence of similar peaks between H2_T72, H1_T72 and H1_T24 was observed in the seriated heat maps (Figure 7B), especially those near 1370, 1421, 1460, 1515 e 1529 cm−1, related to structural carbohydrates (cellulose, xyloglucan), nucleic acids, amino acids and proteins (amides I and II) from the endosperm (Tables 1, 2A and 2B). The main dissimilarity was found at the peak of 2342 and 2360 cm−1 present in H1_T24 and absent in the other samples. These peaks are associated with phosphorus compounds, which showed great intensity in this sample, indicating changes in this compound due to the stress period in this hybrid.
The second group in the HCA was composed by samples of hybrid 2, being H2_T0, H2_T12 and H2_T24 (Figure 7A). The H2_T48 samples were similar to this second group in the HCA due to the peaks associated to carbohydrates, but are distinct from the others due to greater changes in lipids, proteins, amino acids and nucleic acids in this sample, indicating that in the stress period of 0, 12, 24 and 48 h by accelerated ageing did not cause major changes in the biochemical composition of the hybrid endosperm 2. The last group consisted of samples from hybrid 1, where H1_T0 and H1_T12 were also close to H1_T48 (Figure 7A), which were grouped mainly by carbohydrates and proteins (Figure 7B) (Tables 1, 2A and 2B).
Deep analyses showed that there was a small change in the components of the endosperm reserve during accelerated ageing stress for the two hybrids regardless of the level of vigour. The main change along the stress was observed in the samples of H1_T24, where there was an expressive increase in the intensity of the peaks of 2342 and 2360 cm−1. This indicates that, at that time, the high-vigour hybrid required a greater amount of phosphorus compounds, probably to excel in stress and survive to form a normal plant, even under adverse conditions, which was not observed in the endosperm of low-vigour hybrid.
On the other hand, in the embryo spectra, the greatest differences were observed in the time of 72 h for the low-vigour hybrid, where drastic reductions in the intensities of the peaks close to 2880 and 2927 cm−1 were observed, associated to a possible deterioration of lipids, fatty acids and proteins. In addition, there was an increase in the peaks associated with carbohydrates (mono, oligo and polysaccharides) and phosphorus compounds, possibly caused by the increased demand for these components in an attempt to overcome stress. In the high-vigour hybrid, there were no significant changes to the embryo spectra along with the stress, meaning that this hybrid has greater stability in the compounds when subjected to adverse conditions of high temperatures (45 °C) and 100% relative humidity, regardless of the period of exposure.
3.3.2. Partial least squares discriminant analysis (PLS-DA) and model building
PLS-DA is a versatile algorithm that can be used for predictive and descriptive modelling as well as for discriminative variable selection. It is performed in order to sharpen the separation between groups of observations, such that a maximum separation among classes is obtained, and to understand which variables carry the class separating information (Lee et al., 2018, Brereton and Lloyd, 2018, 2014) and it can be used for classification of high dimensional data such as those of ATR-FTIR. Four different models were constructed by using non-variable selection, variable important for projection (VIP), selectivity ratio (SR) and Jack-Knife algorithm for embryonic axis dataset and endosperm dataset respectively. 80% of dataset was used for model calibration and 20% for model validation using 5-fold cross-validation. Data was previously centered and scaled and then submitted to model building.
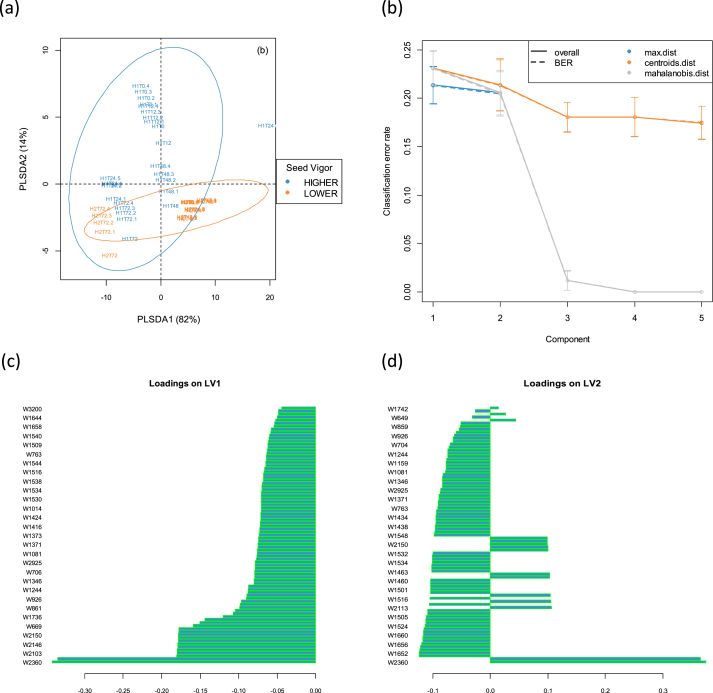
When spectral data were submitted to Partial Least Squares - Discriminant Analysis (PLS-DA) for classification of the samples, better results were found. Figure 8 shows the similarities between the response variable (vigour of seeds) and predictor matrix (Spectral wavenumbers -Figure 8), the number of components to acquire better model performance and the loadings for latent variable 1 and 2 respectively for embryonic axis dataset. In addition, the variables positively or negatively related to Higher and lower vigour seeds can be found in Figure 9(A-B). As it can be observed in Table 3, all models tested performed well, but it was selected the variable selection by VIP which presented better performance of predictors for the embryo samples. The total variance captured by this model was 96%, being 88% for latent variable 1 and 9% for latent variable 2. The model presented better sensitivity and specificity. The model performed well and classified the hybrids better in the level of vigour (high and low vigour). Embryo samples of H2_T72 (low vigour) and H1_T48 (high vigour) were different from the others, meaning that there are structural changes in these samples during the stress time used in this experiment. The loadings plot showed that samples in the latent variable 1 are correlated with carbohydates, and aminoacids and those in latent variable 2 with proteins and peptides.
Figure 8.
PLS-DA score plot (a) followed by the plot (b) with optimal number of components which maximize the model performance and the loadings for latent variable 1 (c) and 2 (d) respectively for embryonic axis dataset.
Figure 9.
PLS-DA coefficients of the maize embryonic axis of lower vigor and higher vigor. (A–B) using variable selection by variable importance for projection (VIP) for variable selection (C–D): PLS-DA coefficients of the maize endosperm of lower vigor and higher vigor.
Table 3.
Statistics of the models tested using non-variable selection, variable importance for projection (VIP) selectivity ratio (SR) and Jack-Knifing algorithm for embryonic and endosperm dataset.
| Maize strucure | Statistics | Non-variable selection | VIP | SR | JK |
|---|---|---|---|---|---|
| Embryonic axis | R2X | 96.95 | 98.79 | 97.11 | 98.82 |
| R2Y | 68.48 | 70.42 | 70.01 | 65.90 | |
| Sensitivity | 1 | 1 | 1 | 1 | |
| Specificity | 1 | 1 | 1 | 1 | |
| Endosperm | R2X | 96.55 | 93.14 | 96.88 | 80.65 |
| R2Y | 54.76 | 92.02 | 54.43 | 96.77 | |
| Sensitivity (True positive) | 0.85 | 1 | 0.85 | 1 | |
| Specificity (True Negative) | 0.81 | 1 | 0.81 | 1 |
A similar analysis for the endosperm dataset can be found in Figure 10, together with the loadings. Variables correlated with higher and lower vigour maize hybrids are also found in Figure 9 (C-D). The total variance captured in the latent variables was 96%, being 82% and 14% for the latent variables 1 and 2, respectively. The duration of stress did not cause differentiated changes in all hybrids (low and high vigour), and they were grouped together. Small changes occur for H1_T0 (not stressed) and at the time of 12 h of stress duration (H1_T12), although these changes are minimal, not interfering in the clustering of PLS-DA. The confusion matrix is presented in Table 4.
Figure 10.
PLS-DA score plot (a) followed by the plot (b) with optimal number of components which maximize the model performance and the loadings for latent variable 1 (c) and 2 (d) respectively for endosperm.
Table 4.
The confusion matrix for the models where the sensitivity and specificity was lower than 1 and the misclassification error of the models related to the confusion matrix.
| Observed | Predicted |
|||
|---|---|---|---|---|
| Higher Vigor | Lower Vigor | %Correct | Total | |
| Higher Vigor | 17 | 4 | 17 | 21 |
| Lower Vigor | 3 | 17 | 17 | 20 |
| Total | 20 | 21 | 34 | 41 |
∗Misclassification error = 0.17.
H2 samples (0, 12, 24 and 48 h) were similar and close to the H1_T48 samples. The H2_T72 samples were those that underwent major alterations, being separated from the others, although these alterations were small in the endosperm. The model also presented better performance and the response variable (seed vigour) showed a higher correlation (with the 3 predicted latent variables that are necessary to maximize covariance between the predictors (x) and the response variable (y). For endosperm, phosphorus compounds and aminoacids were the main chemical groups classifying the samples.
4. Conclusions
The study prompts us to conclude that ATR-FTIR combined with chemometrics are powerful tools for screening the physiological quality of hybrid maize seeds and to predict the seed vigour of the samples and provides a theoretical basis for the genetic improvement of maize cultivars that aim at higher physiological seed quality.
High-vigour seeds undergo minimal changes in biochemical composition during stress by accelerated ageing, evidencing the relation of the compounds with the vigour of the seeds. Low-vigour seeds are more sensitive to stress and this lower tolerance is associated with reduced lipid and protein content and increased amino acids, carbohydrates and phosphorus compounds in the embryo.
High-vigour seeds show an increase in peaks associated with amino acids and phosphorous compounds in the endosperm after 24 h of stress. Low-vigour seeds present these high-intensity peaks only after 72 h in the embryo. For endosperm, phosphorus compounds and aminoacids were the main chemical groups classifying the samples.
Declarations
Author contribution statement
G. Camargo Andrade, C. M. Medeiros Coelho: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
V. Gavicho Uarrota: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by CONICYT-FONDECYT Project 3190055, Chile, FAPESC (Fundação e Amparo à Pesquisa e Inovação do Estado de Santa Catarina) and CAPES (Coordenação de perfeiçoamento de Pessoal de Nível Superior).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Andrade G.C., Coelho C.M.M., Padilha M.S. Seed reserves reduction rate and reserves mobilization to the seedling explain the vigour of maize seeds. J. Seed Sci. 2019;41(4):488–497. [Google Scholar]
- Association of Official Seed Analysts . AOSA; Lincoln: 1983. Seed Vigor Testing Handbook. [Google Scholar]
- Baker M.J., Trevisan J., Bassan P., Bhargava R., Butler H.J., Dorling K.M., Fielden P.R., Fogarty S.W., Fullwppd N.J., Heys K.A., Hughes C., Lasch P., Martin-Hirsch P.L., Obinaju B., Sockalingum G.D., Sulé-Susso J., Strong R.J., Walsh M.J., Wood B.R., Gardner P., Martin F.L. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014;9(8):1771–1791. doi: 10.1038/nprot.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareke T. Biology of seed development and germination physiology. Adv. Plants Agric. Res. 2018;8(4):336. [Google Scholar]
- Barreto L., Garcia Q. Accelerated ageing and subsequent imbibition affect seed viability and the efficiency of antioxidant system in macaw palm seeds. APP (Acta Physiol. Plant.) 2017;39(3) [Google Scholar]
- Bewley J.D., Bradford K.J., Hilhorst H.W.M., Nonogaki H. third ed. Springer; New York: 2013. p. 392. (Seeds: Physiology of Development, Germination and Dormancy). [Google Scholar]
- Buescher J.M., Driggers E.M. Integration of omics: more than the sum of its parts. Canc. Metabol. 2016;4(1) doi: 10.1186/s40170-016-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brereton R., Lloyd G. Partial least squares discriminant analysis: taking the magic away. J. Chemometr. 2014;28(4):213–225. [Google Scholar]
- Brereton R., Lloyd G. Partial least squares discriminant analysis for chemometrics and metabolomics: how scores, loadings, and weights differ according to two common algorithms. J. Chemometr. 2018;32(4) [Google Scholar]
- Carvalho N.M., Nakagawa J. 5.ed. FUNEP; Jaboticabal: 2012. p. 590. (Sementes: ciência, tecnologia e produção). [Google Scholar]
- Černá M., Barros A.S., Nunes A., Rocha S.M., Delgadillo I., Čopíková J., Coimbra M.A. Use of FT-IR spectroscopy as a tool for the analysis of polysaccharide food additives. Carbohydr. Polym. 2003;51(4):383–389. [Google Scholar]
- Coelho C.M.M., Benedito V.A. Seed development and reserve compound accumulation in common bean (Phaseolus vulgaris L.) Seed Sci. Biotechnol. 2008;2(2):42–52. [Google Scholar]
- Corbineau F. Markers of seed quality: from present to future. Seed Sci. Res. 2012;22(S1):S61–S68. [Google Scholar]
- Čopíková J., Barros A.S., Šmidová I., Černá M., Teixeira D.H., Delgadillo I. Influence of hydration of food additive polysaccharides on FT-IR spectra distinction. Carbohydr. Polym. 2006;63:355–359. [Google Scholar]
- Cozzolino D., Roumeliotis S., Eglinton J. Evaluation of the use of attenuated total reflectance mid infrared spectroscopy to determine fatty acids in intact seeds of barley (Hordeum vulgare) LWT-Food Sci. Technol. 2014;56:478–483. [Google Scholar]
- Cozzolino D., Roumeliotis S., Eglinton J. Feasibility study on the use of attenuated total reflectance infrared spectroscopy as high throughput screening tool to phenotype single barley seeds (Hordeum vulgare L.) Biosyst. Eng. 2013;116:379–384. [Google Scholar]
- de Bittencourt Sonia Regina Mudrovitsch, Vieira Roberval Daiton. Temperatura e período de exposição de sementes de milho no teste de envelhecimento acelerado. Rev. Bras. Sementes. 2006:161–168. [Google Scholar]
- Doria E., Galleschi L., Calucci L., Pinzino C., Pilu R., Cassani E., Nielsen E. Phytic acid prevents oxidative stress in seeds: evidence from a maize (Zea mays L.) low phytic acid mutant. J. Exp. Bot. 2009;60(3):967–978. doi: 10.1093/jxb/ern345. [DOI] [PubMed] [Google Scholar]
- Finch-Savage W.E., Bassel G.W. Seed vigour and crop establishment: extending performance beyond adaptation. J. Exp. Bot. 2015;67(3):567–591. doi: 10.1093/jxb/erv490. [DOI] [PubMed] [Google Scholar]
- Gu J., Hou D., Li Y., Chao H., Zhang K., Wang H., Xiang J., Raboanatahiry N., Wang B., Li M. Integration of proteomic and genomic approaches to dissect seed germination vigor in Brassica napus seeds differing in oil content. BMC Plant Biol. 2019;19(1) doi: 10.1186/s12870-018-1624-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez G., Cruz F., Moreno J., González-Hernández V.A., Vázquez-Ramos J.M. Natural and artificial seed ageing in maize: germination and DNA synthesis. Seed Sci. Res. 1993;3(4) [Google Scholar]
- Hampton J., Conner A., Boelt B., Chastain T., Rolston P. Climate change: seed production and options for adaptation. Agriculture. 2016;6(3):33. [Google Scholar]
- Han C., Zhen S., Zhu G., Bian Y., Yan Y. Comparative metabolome analysis of wheat embryo and endosperm reveals the dynamic changes of metabolites during seed germination. Plant Physiol. Biochem. 2017;115:320–327. doi: 10.1016/j.plaphy.2017.04.013. [DOI] [PubMed] [Google Scholar]
- Kuhnen S., Ogliari J.B., Dias P.F., Boffo E.F., Correia I., Ferreira A.G., Delgadillo I., Maraschin M. ATR-FTIR spectroscopy and chemometric analysis applied to discrimination of landrace maize flours produced in southern Brazil. Int. J. Food Sci. Technol. 2010;45(8):1673–1681. [Google Scholar]
- Kumar S., Lahlali R., Liu X., Karunakaran C. Infrared spectroscopy combined with imaging a new developing analytical tool in health and plant science. Appl. Spectrosc. Rev. 2016 [Google Scholar]
- Lee L., Liong C., Jemain A. Partial least squares-discriminant analysis (PLS-DA) for classification of high-dimensional (HD) data: a review of contemporary practice strategies and knowledge gaps. The Analyst. 2018;143(15):3526–3539. doi: 10.1039/c8an00599k. [DOI] [PubMed] [Google Scholar]
- Li-Chan E.C.Y. Introduction to vibrational spectroscopy in food science. In: Li-Chan E.C.Y., Griffiths P.R., Chalmers J.M., editors. Vol. 1. John Willey & Sons Ltd; United Kingdom: 2010. pp. 3–29. (Vibrational Spectroscopy in Food Science). [Google Scholar]
- Lin L., Ockenden I., Lott J.N. The concentrations and distribution of phytic acid-phosphorus and other mineral nutrients in wild-type and low phytic acid1-1 (lpa1-1) corn (Zea mays L.) grains and grain parts. Can. J. Bot. 2005;83(1):131–141. [Google Scholar]
- Lopes W.A., Fascio M. Esquema para interpretação de espectros de substâncias orgânicas na região do infravermelho. Quím. Nova. 2004;27(4):670–673. [Google Scholar]
- López-Sánchez M., Ayora-Cañada M.J., Molina-Díaz A. Olive fruit growth and ripening as seen by vibrational spectroscopy. J. Agric. Food Chem. 2010;58(1):82–87. doi: 10.1021/jf902509f. [DOI] [PubMed] [Google Scholar]
- Marcos Filho J. Seed vigor testing: an overview of the past, present and future perspective. Sci. Agric. 2015;72(4):363–374. [Google Scholar]
- Marcos Filho . J. Testes de vigor: importância e utilização. In: Krzyzanowski F.C., Vieira R.D., França Neto J.B., editors. Vigor de sementes: conceitos e testes. Londrina: Abrates; 1999. pp. 1–21. [Google Scholar]
- Nerling D., Coelho C.M.M., Brümmer A. Biochemical profiling and its role in physiological quality of maize seeds. J. Seed Sci. 2018;40(1):7–15. [Google Scholar]
- Nerling Daniele. Diversidade genética para qualidade fisiológica de sementes produzidas por cruzamentos intervarietais de milho (Zea mays L.) J. Seed Sci. 2013;35(4) [Google Scholar]
- Oliveira G.E., Pinho R.G.V., de Andrade T., de Pinho É.V.R.V., dos Santos C.D., Veiga A.D. Physiological quality and amylase enzyme expression in maize seeds. Cienc. E Agrotecnol. 2013;37(1):40–48. [Google Scholar]
- Oliveira R.N., Mancini M.C., de Oliveira F.C.S., Passos T.M., Quilty B., da Thiré R.M.S.M., McGuinness G.B. FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Materia. 2016;21(3):767–779. [Google Scholar]
- Prazeres C.S., Coelho C.M.M. Genetic divergence and heterosis related to physiological quality in maize seeds. Bragantia. 2016;75(4):411–417. [Google Scholar]
- Prazeres Camila Segalla, Coelho Cileide Maria Medeiros. Heterose para qualidade fisiológica de sementes na obtenção de híbridos de milho. Revista Brasileira de Milho e Sorgo. 2016;15(1):124–133. 2016. [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2019. R: A Language and Environment for Statistical Computing. Disponível em: https://www.R-project.org/ [Google Scholar]
- Rajjou L., Duval M., Gallardo K., Catusse J., Bally J., Job C., Job D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012;63(1):507–533. doi: 10.1146/annurev-arplant-042811-105550. [DOI] [PubMed] [Google Scholar]
- Savitzky A., Golay M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964;36(8):1627–1639. [Google Scholar]
- Sbrussi C.A.G., Zucareli C. Germinação de sementes de milho com diferentes níveis de vigor em resposta à diferentes temperaturas. Semina Ciências Agrárias. 2014;35(1):215. [Google Scholar]
- Schulz H., Baranska M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007;43(1):13–25. [Google Scholar]
- Silverstein R.M., Webster F.X., Kiemle D.J. seventh ed. John Wiley and Sons; Hoboken, NJ: 2005. Spectrometric Identification of Organic Compounds. [Google Scholar]
- Socrates G. third ed. J. Wiley and Sons; Chichester: 2001. p. 348. (Infrared and Raman Characteristic Group Frequencies: Tables and Charts). [Google Scholar]
- Suresh A., Shah N., Kotecha M., Robin P. Evaluation of biochemical and physiological changes in seeds of Jatropha curcas L. under natural aging, accelerated aging and saturated salt accelerated aging. Sci. Hortic. 2019;255:21–29. [Google Scholar]
- Tian P.-P., Lv Y.-Y., Yuan W.-J., Zhang S.-B., Hu Y.-S. Effect of artificial aging on wheat quality deterioration during storage. J. Stored Prod. Res. 2019;80:50–56. [Google Scholar]
- Uarrota V., Moresco R., Coelho B., Nunes E., Peruch L., Neubert E., Rocha M., Maraschin M. Metabolomics combined with chemometric tools (PCA, HCA, PLS-DA and SVM) for screening cassava (Manihot esculenta Crantz) roots during postharvest physiological deterioration. Food Chem. 2014;161:67–78. doi: 10.1016/j.foodchem.2014.03.110. [DOI] [PubMed] [Google Scholar]
- Uarrota V.G., Amante E.R., Demiate I.M., Vieira F., Delgadillo I., Maraschin M. Physicochemical, thermal, and pasting properties of flours and starches of eight Brazilian maize landraces (Zea mays L.) Food Hydrocolloids. 2013;30(2):614–624. [Google Scholar]
- Uarrota V.G., Rocha M., Maraschin M. Non-targeted metabolomic profiling of maize landraces (Zea mays l.) combined with chemometric tools. Int. J. Biochem. Res. Rev. 2017;20(1-IJBCRR. 35832):1–9. [Google Scholar]
- Uarrota V.G., Segatto C., Voytena A.P.L., Maraschin M., Avila L.V., Kazama D.C.S., Coelho C.M.M., Souza C.A. Metabolic fingerprinting of water-stressed soybean cultivars by gas chromatography, near-infrared and UV-visible spectroscopy combined with chemometrics. J. Agron. Crop Sci. 2018 [Google Scholar]
- Van Soest J.J., Tournois H., de Wit D., Vliegenthart J.F. Short-range structure in (partially) crystalline potato starch determined with attenuated total reflectance Fourier-transform IR spectroscopy. Carbohydr. Res. 1995;279:201–214. [Google Scholar]
- Ventura L., Donà M., Macovei A., Carbonera D., Buttafava A., Mondoni A., Rossi G., Balestrazzi A. Understanding the molecular pathways associated with seed vigor. Plant Physiol. Biochem. 2012;60:196–206. doi: 10.1016/j.plaphy.2012.07.031. [DOI] [PubMed] [Google Scholar]
- Wen D., Hou H., Meng A., Meng J., Xie L., Zhang C. Rapid evaluation of seed vigour by the absolute content of protein in seed within the same crop. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-23909-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Ning F., Hu X., Wang W. Genetic modification for improving seed vigor is transitioning from model plants to crop plants. Front. Plant Sci. 2017;8 doi: 10.3389/fpls.2017.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia F., Chen L., Sun Y., Mao P. Relationships between ultrastructure of embryo cells and biochemical variations during ageing of oat (Avena sativa L.) seeds with different moisture content. Acta Physiol. Plant. 2015;37(4) [Google Scholar]
- Yan D., Duermeyer L., Leoveanu C., Nambara E. The functions of the endosperm during seed germination. Plant Cell Physiol. 2014;55(9):1521–1533. doi: 10.1093/pcp/pcu089. [DOI] [PubMed] [Google Scholar]
- Zhang A., Sun H., Wang P., Han Y., Wang X. Modern analytical techniques in metabolomics analysis. The Analyst. 2012;137(2):293–300. doi: 10.1039/c1an15605e. [DOI] [PubMed] [Google Scholar]
- Zhao M., Zhang H., Yan H., Qiu L., Baskin C.C. Mobilization and role of starch, protein, and fat reserves during seed germination of six wild grassland species. Front. Plant Sci. 2018;9 doi: 10.3389/fpls.2018.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]