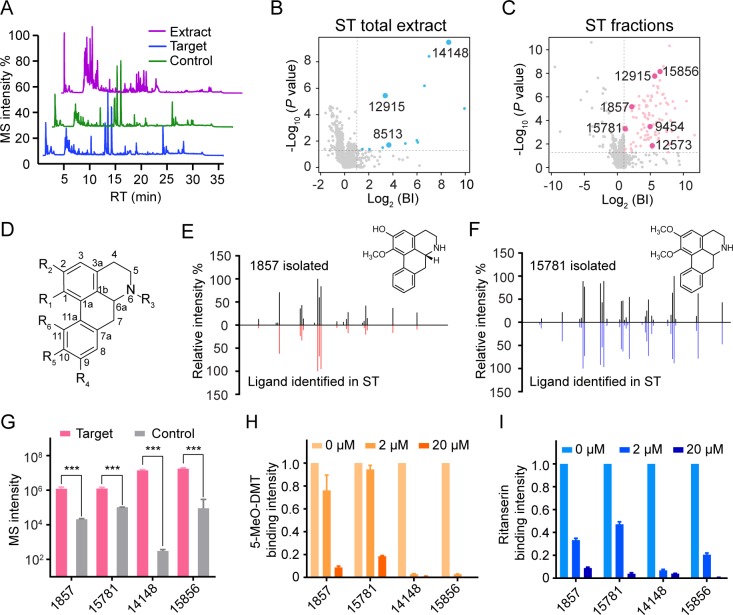
Figure 2.
Identification of aporphines active at 5-HT2C from crude and fractionated extracts of Stephania tetrandra (ST). (A) Representative LC-MS chromatograms of ST crude extract, 5-HT2C target, and control. (B, C) Initial hits from screening ST crude extract (blue dots) or ST extract fractions (pink dots) by affinity MS combined with metabolomics. Aporphines are annotated with larger dots. BI, binding index. (D) Scaffold of the aporphines identified in this study (specific structures listed in Table S3). (E, F) Structural validation of 1857 and 15781 by MSMS analysis. (G) Validation of ligand binding to purified 5-HT2C by pure compounds using the affinity MS binding assay. The MS intensity of each ligand was significantly higher in the 5-HT2C target than in control (***P < 0.001, n = 4). (H, I) Competition of 5-MeO-DMT or ritanserin binding to purified 5-HT2C with increasing concentrations of each aporphine. MS intensity of 5-MeO-DMT or ritanserin bound to purified 5-HT2C was normalized to that in the absence of any aporphine. Data were obtained from two independent experiments in technical duplicate. Error bars represent SEM.