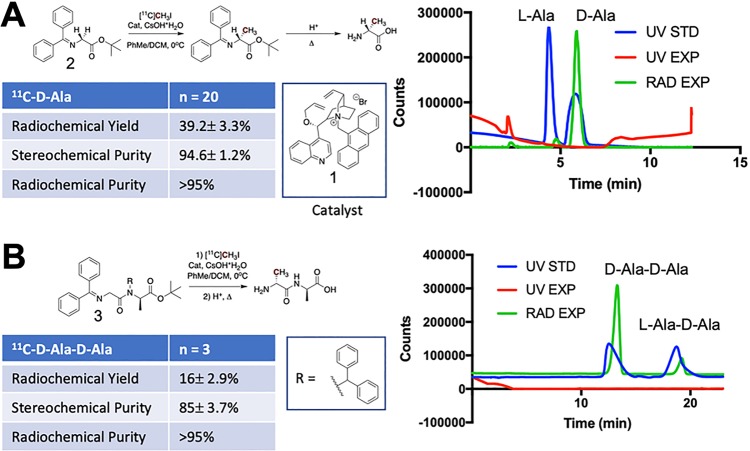
Figure 2.
Radiochemical syntheses of d-[11C]ala and d-[11C]ala–d-ala using a chiral, phase-transfer cinchonidinium-derived catalyst. (A) High enantiomeric excess synthesis of d-[11C]ala via asymmetric alkylation of a glycine-derived Schiff base with [11C]CH3I. The analytical HPLC data on the right shows the radioactive trace of d-[11C]ala (green) overlaid with a racemic sample of alanine (blue) with both d- and l-enantiomers present in equal concentrations. (B) An analogous procedure was used for the radiosynthesis of d-[11C]ala–d-ala at approximately 70% diastereomeric excess. The analytical HPLC data on the right show the radioactive trace of d-[11C]ala–d-ala (green) overlaid with a sample containing d-ala–d-ala and the undesired diastereomer l-ala–d-ala present in equal concentrations (blue).