Abstract
There is growing interest in relationships between borderline personality disorder (BPD) pathology and physical health outcomes. Diagnostic BPD and BPD-related traits, for instance, have been shown to associate with self-reported cardiovascular disease and various cardiometabolic risk factors. However, potential confounding of these associations by comorbid depression, which itself contributes to risk for heart disease, remains unresolved, and prior research is limited by nearly uniform reliance on self-reported health status. In the present study, we examine the association of BPD traits and contemporaneously assessed depressive mood with instrumented measures of cardiometabolic risk in a midlife community sample (N = 1,295). BPD pathology was measured using dimensional, multi-informant trait measures; depressive symptomology was self-reported; and cardiometabolic risk was indexed via multiple indicators of insulin resistance, adiposity, dyslipidemia, and blood pressure. Structural equation modeling was used to estimate the effects of BPD traits and depressive symptoms on aggregated cardiometabolic risk, adjusting for their shared variance. Results showed both BPD pathology features and depressive symptomatology related to extent of cardiometabolic risk; when examined simultaneously, only BPD associated independently with risk indicators. In further supporting a link between BPD pathology and cardiovascular disease risk, these findings warrant future work to elucidate intervening behavioral and biological mechanisms.
Although borderline personality disorder (BPD) is well-studied for its relation to psychosocial impairments, recent research suggests this same pathology may also contribute to physical health risks (Dixon-Gordon et al., 2015; Quirk et al., 2016; Tomko et al., 2014). For instance, diagnostic BPD or BPD-related traits have been found associated with both self-reported cardiovascular disease (Lee et al., 2010; Moran et al., 2007; El-Gabalawy et al., 2010; Powers & Oltmanns, 2013) and various heart disease risk factors, such as obesity (Frankenburg & Zanarini, 2004; Greggersen et al., 2011a, 2011b; Powers & Oltmanns, 2013; Sansone et al., 2001), hypertension (Frankenburg & Zanarini, 2004; El-Gabalawy et al., 2010), fasting insulin or diabetes (Greggersen et al., 2011a; El-Gabalawy et al., 2010), and preclinical atherosclerosis (carotid artery thickening) (Greggersen et al., 2011a). In addition to studying risk factors individually, the metabolic syndrome—a composite measure of abnormalities in lipid metabolism, glycemic control, central adiposity, and blood pressure—is commonly used to represent cardiovascular risk and predict incident disease. Using this index, one additional study found that prevalence of the metabolic syndrome among BPD patients from an inpatient psychiatric sample exceeded that of the general population (Kahl et al., 2013). These investigators also reported that BPD patients with the metabolic syndrome were more likely than those without to have co-occurring depression and dysthymia, but did not address whether this comorbidity fully confounded the association between BPD and metabolic syndrome.
The confound of comorbid depression is particularly relevant because depressive symptoms are known to confer risk for cardiovascular disease and metabolic syndrome (Rugilies, 2002; Pan et al., 2011; Vancampfort et al., 2014). It is possible that associations with depression and BPD are due to a more general, shared pathway, such as trait neuroticism or emotion dysregulation, which have established associations with cardiovascular risk factors (Bliel et al., 2008; Kinnunen et al., 2005; Gianaros et al., 2014). Behaviorally, both forms of psychopathology involve negative affectivity and problematic health behaviors (e.g., poor diet, reduced physical activity, and substance use; Strine et al., 2008; Bonnet et al., 2005; Trull et al., 2018), that contribute to risk for incident cardiovascular disease (Patnode et al., 2017). Biological explanations for the association between depression and cardiovascular disease risk may also extend to BPD, including the potential role of systemic inflammation (Schiepers et al., 2005; Marsland et al., 2010), perturbed adrenocortical functioning (Anagnostis et al., 2009), and altered cardiac autonomic control (Kop et al., 2010; Carnethon et al., 2006). However, unlike depression, BPD is further defined by hostility, impulsivity, and interpersonal problems (American Psychiatric Association, 2013) that are themselves associated with cardiovascular outcomes (e.g., Boggs & Roberts, 2004; Valtorta et al., 2016) and could confer risk independent of covarying depression or common vulnerability factor.
At present, the unique effects of BPD features are unclear. Among the few studies that controlled for mood disorders, only one measured concurrent symptomatology (Sansone et al., 2001) and the remainder relied on self-reported lifetime occurrence. Of these, four reported that associations between BPD measures and participant-reported history of cardiovascular disease or individual risk factors persisted after adjusting for lifetime depression (Lee et al., 2010; El-Gabalawy et al., 2010; Powers & Oltmanns, 2013; Sansone et al., 2001), whereas two studies failed to find a unique effect of BPD (Greggersen et al., 2011a, 2011b). Given this inconclusive literature and its nearly uniform reliance on self-reported health status, as opposed to measured cardiovascular risk, here we further examine BPD features in relation to aggregated cardiometabolic risk and contemporaneously assessed depressive mood. The study uses data from a large community sample of midlife adults on whom instrumented measures of component risk factors were available, along with a multi-informant, dimensional measure of BPD traits and reported depressive symptomatology.
Method
Data for this study were acquired from the University of Pittsburgh Adult Health and Behavior (AHAB) project, a registry of behavioral and biological measurements on non-Hispanic Causcasian and African American individuals (30–54 years old) recruited in 2001–2005 via mass-mail solicitation from communities of southwestern Pennsylvania in the United States (Manuck et al., 2010; Marsland et al., 2010). Exclusion criteria included history of atherosclerotic, cardiovascular, chronic kidney, or liver disease; past-year cancer treatment; neurologic disorders; and psychotic illness. Further exclusions included pregnancy and using insulin, nitrates, glucocorticoid, antiarrhythmic, psychotropic, or prescription weight-loss medications. Informed consent was obtained in accordance with approved protocol guidelines of the University of Pittsburgh Institutional Review Board. Data used for the present analyses were available for 1,295 participants, which is the final sample size for this study. The demographic breakdown is presented in Table 1.
Table 1.
Descriptive statistics of psychopathology and biomedical characteristics
| Mean (SD) or % | ||
|---|---|---|
| Demographics | ||
| Age (years) | 44.6 (6.7) | |
| Sex (male) | 47.3 | |
| Race (Caucasian) | 83.5 | |
| Years of education | 15.7 (2.8) | |
| Annual family income | Less than $14,999 | 10.7 |
| $15,000 – 24,999 | 9.5 | |
| $25,000 – 49,999 | 26.8 | |
| $50,000–64,999 | 16.8 | |
| Above $65,000 | 35.5 | |
| Psychopathology and Biomedical Measures | ||
| BPD traits | ||
| Self | 1.40 (1.18) | |
| Informant x | 1.41 (0.51) | |
| Informant y | 1.34 (0.48) | |
| Depression | ||
| Somatic Symptoms | 0.16 (0.25) | |
| Anhedonia | 0.25 (0.29) | |
| Depressive Cognitions | 0.18 (0.31) | |
| Metabolic Syndrome | ||
| Systolic BP (mm Hg) | 116.44 (13.6) | |
| Diastolic BP (mm Hg) | 78.31 (9.4) | |
| Insulin (μU/ ml) | 13.42 (7.7) | |
| Glucose (mg/dl) | 96.45 (18.2) | |
| BMI (kg/ m2) | 27.46 (5.8) | |
| Waist circumference (in.) | 91.95 (15.9) | |
| HDL (mg/dl) | 53.52 (14.6) | |
| Triglycerides (mg/dl) | 120.48 (81.3) | |
Note. BPD = borderline personality disorder; BP = blood pressure; BMI = body mass index; HDL = high-density lipoprotein. BPD traits measured using the NEO Five-Factor Inventory; Depression measured using the Beck Depression Inventory.
BPD Assessment.
A composite measure of scores on items of the NEO Five-Factor Inventory (NEO-FFI; Costa & McCrae, 1992) was used to measure BPD. These items selected to be prototypical of BPD have demonstrated sufficient convergent and discriminative validity to support their use for approximating its symptomology (Few et al., 2016). Participants and up to two informants (88% had two informants) rated the relevant personality items from the NEO-FFI. Dimensional BPD pathology scores were calculated from the relevant items as described in Few and colleagues (2016) for the self- and informant-reported items.
Depression Assessment.
Depressive symptoms were self-reported using the Beck Depression Inventory (BDI, Beck, Steer & Garbin, 1988). Sub-scale scores for Anhedonia, Depressive Cognitions, and Somatic Symptoms (Shafer, 2006) were used.
Cardiometabolic Assessment.
Presence of the metabolic syndrome is commonly defined by a consensus count of the number of syndrome components whose values exceed thresholds deemed indicative of elevated disease risk by various professional bodies (e.g., American Heart Association, National Cholesterol Education Program, International Diabetes Federation). As a binary measure, the metabolic syndrome has at least two notable limitations: 1) dichotomizing distributions of continuous variables like blood pressure and fasting glucose loses much predictive information; and 2) in younger, midlife populations, true risk may be underestimated when many individuals exhibit subthreshold elevations on multiple indicators (Dermody et al., 2016). Also, a quantitative index of cardiometabolic risk created from continuous distributions of the same variables better predicts incident disease than categorically defined metabolic syndrome (Agarwal et al., 2012), and confirmatory factor analyses using multiple risk variables consistently show a single second-order factor underlying four first-order factors (blood pressure, insulin resistance, weight/adiposity, and dyslipidemia) (Shen et al., 2003; Marsland et al., 2010). Here, we express relative cardiometabolic risk (CMR) by derivation of this second-order latent factor using the same hierarchical modeling techniques.
Components of CMR were assessed in the morning after a 12-hour overnight fast, as detailed by Manuck et al. (2010). Blood pressure was measured by sphygmomanometry as the mean of two consecutive readings obtained in a seated position. At this visit, a nurse completed a medical history interview, determined BMI (kg/m2), and drew a 40 ml blood sample. Determination of fasting serum lipids, glucose, and insulin was performed by the Heinz Nutrition Laboratory, University of Pittsburgh Graduate School of Public Health, as described previously (Muldoon et al., 2000).1
Results
All statistical models were estimated using Mplus 8.3 (Muthén & Muthén, 2019). The effect of sex, age, and race was adjusted for at the level of observed variables in every model. Descriptive statistics for study variables can be found in Table 1.
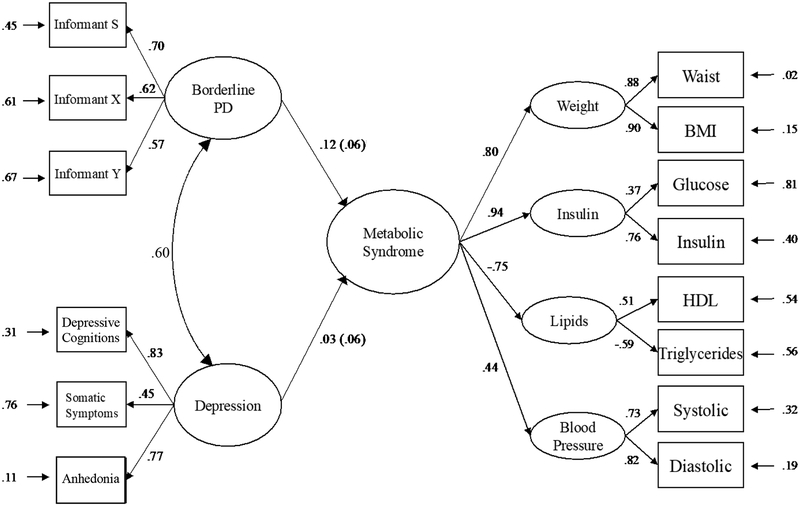
CMR was modelled following prior work in this sample (Marsland et al., 2010; McCaffery et al., 2012). Four first-order latent factors with two indicators each were specified using confirmatory factor analysis (CFA): elevated blood pressure (indicated by systolic and diastolic pressures), insulin resistance (fasting insulin and glucose concentrations), heightened adiposity (BMI and waist circumference), and dyslipidemia (fasting high-density lipoprotein [HDL] and triglycerides). Good overall fit for this CMR model using this these data has been previously reported (Dermondy et al., 2016). A latent factor for BPD was estimated from self and informant ratings of FFM traits using a multi-informant modeling approach, which leverages shared variance between raters to improve measurement reliability (DeYoung, 2006). Informant and self-report ratings all showed significant factor loadings on the latent BPD factor. BDI subscale scores were then used to estimate a latent depression variable. All factor loadings were significant for the BPD and depression factors, and both models were fully saturated. Once each of these models was estimated individually, all three latent factors were combined into a single CFA to determine overall model fit, along with the zero-order correlations between latent BPD, depression, and CMR. This combined model achieved good fit by all three alternative fit indices (χ2 = 182.64, df = 70; RMSEA = .04; CFI = .98; SRMR = .02). As expected, BPD and depression each had significant associations with CMR (r = .15, p < .001; r = .11, p = .007). Furthermore, BPD and depression were strongly correlated (r = .60, p <.001) supporting the need for the subsequent multivariate model to account for covariation.
For the final model, we regressed CMR on BPD and depression simultaneously to evaluate the effects of non-shared variance (depicted in Figure 1). This model had the same number of parameters and equivalent fit to the combined CFA. When CMR was regressed onto BPD adjusting for the effect of depression, the standardized beta only decreased by .03 and remained significant (β = .12, p = .031). In contrast, the effect of CMR on depression controlling for BPD decreased by .08 and was no longer significant (β = .03, p = .568).
Figure 1. Structural model of metabolic syndrome regressed on BPD traits and depression.
Note. All parameter estimates presented in standardized form. BMI = body mass index; HDL = high-density lipoprotein; BP = blood pressure; Informant S= Self-report, Informants X and Y: other informant reports.
At request of a reviewer, we added socioeconomic status (measured using reported family income and years of education) as a covariate at the level of observed variables. This addition did not change results of the correlational model. In the regression model, the association between depression and CMR remained non-significant (β=.02, p =.682), while the effect of BPD on CMR also attenuated by .01, but was no longer significant (p = .050). Indicators of socioeconomic status associated modestly with CMR indicators (βs ≤ |.05|), whereas income showed much stronger associations with BPD traits (βs ≥ −.18).
Discussion
In a contribution to the growing literature establishing a link between BPD pathology and cardiovascular disease risk, we aimed to evaluate whether this association exists independently of comorbid depressive symptomology. Consistent with previous research, results indicate that both BPD traits and depressive pathology predicted CMR. However, only the effect of BPD traits remained significant and virtually unchanged after accounting for their shared variance, affirming its unique role over and above co-occurring depression.
Having more firmly established a broad link, this study provides impetus for further investigation of potential pathways that may account for the relationship between BPD pathology and CMR. The strong correlation between BPD traits and depression found in this study, within the context of extensive clinical comorbidity, reinforces the possibility of a shared source of pathology that contributes to risk in each. Our study points to the value in distinguishing general and specific risk factors, and the need to account for overlapping features when designing studies of BPD and CMR to clarify processes underlying their association. In addition to shared vulnerability factors, the role of BPD features distinct from depression should be evaluated in future work. Precision gained by examining relationships between specific components of CMR and BPD symptoms involving impulsivity, anger, and interpersonal dysfunction could inform mechanistic models. Another intriguing result was the minor impact on the association between CMR and BPD traits after adjusting for socioeconomic status. This was not due to large direct effects of socioeconomic status on CMR, but rather to relatively large associations with BPD traits. Exploring the relationship between BPD and socioeconomic status could be informative for understanding their joint role in CMR. The additional variance in cardiovascular risk associated with BPD traits independent of shared depressive symptoms could also be a function of severity rather than kind of psychopathology. Thus, it’s possible that BPD pathology involves a greater breadth of pathological features that have an additive effect on disease risk. Indeed, itis consistently found that the presence of personality pathology negatively affects psychotherapeutic and pharmacological treatment outcomes of depression (Levenson et al., 2012), so it is reasonable to consider this effect in the context of physical health outcomes.
Regardless of how BPD traits relate to CMR, the effect found in this study suggests a clinically meaningful relationship. To compare with other intervening variables a medical professional commonly considers, each standard deviation increase in BPD traits has the same effect on CMR as approximately 9.2 years of aging in the sample used for this study. The effect of BPD traits on CMR is large enough that clinicians treating patients with these features should recommend monitoring cardiovascular health.
Some limitations to the current study must be noted. Given the relationship between cardiovascular risk and age (North & Sinclair, 2012), it is possible that findings in this sample of mid-life adults do not generalize to other periods of development. However, our results are consistent with previous studies of BPD using samples representing a range of ages (e.g. El-Gabalwy et al., 2010). Replication of our results with different racial and ethnic groups, and using different measures of BPD and depression are also needed to assess their generalizability. Additionally, though our use of a multi-informant trait measure of BPD is a methodological strength, some reservation is warranted in direct comparison with the single-informant measure of depression. Unlike previous studies, we did not limit analyses to a particular threshold of clinical severity for either BPD pathology or CMR. It is possible that full syndrome manifestation of BPD or recruitment of a sample enriched for the presence of individuals at high risk for cardiovascular disease would yield even stronger associations. By capturing a continuum of severity in BPD traits, the relationship found with CMR, even at sub-clinical levels, arguably provides more compelling evidence for this association and could be useful for conceptualizing disease prevention.
Findings from this study underscore the importance of widening the scope of BPD research to include physical health processes and outcomes in addition to psychosocial dysfunction. Understanding the relationship between BPD pathology and cardiovascular disease risk has implications for direct clinical application, as well as the potential to enrich theories of the interface between physical and psychological functioning.
Acknowledgments
This research was supported by a grant from the National Institute of Health (PO1 HL040962, PI: Manuck). The views contained in the manuscript are those of the authors and not necessarily those of the funding source.
Footnotes
A sensitivity analysis was performed to evaluate the effect of including participants taking medications that affect cardiovascular measurements used to estimate CMR. Exclusion of participants taking antihypertensives, oral hypoglycemics and cholesterol-lowering medications resulted in 146 dropped cases (n = 1,153). Results from this model were virtually identical, so findings from the full sample are reported.
Declarations of interest: None.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Agarwal S, Jacobs DR Jr, Vaidya D, Sibley CT, Jorgensen NW, Rotter JI, … Herrington DM (2012). Metabolic syndrome derived from principal component analysis and incident cardiovascular events: The multi ethnic study of atherosclerosis (MESA) and health, aging, and body composition (health ABC). Cardiology Research and Practice, 1(1), 919425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, & Mikhailidis DP (2009). The pathogenetic role of cortisol in the metabolic syndrome: A hypothesis. The Journal of Clinical Endocrinology & Metabolism, 94(8), 2692–2701. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Garbin MG (1988). Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review, 8(1) 77–100. [Google Scholar]
- Bleil ME, Gianaros PJ, Jennings JR, Flory JD, & Manuck SB (2008). Trait negative affect: Toward an integrated model of understanding psychological risk for impairment in cardiac autonomic function. Psychosomatic Medicine, 70(3), 328–337. [DOI] [PubMed] [Google Scholar]
- Bogg T, & Roberts BW (2004). Conscientiousness and health-related behaviors: A meta-analysis of the leading behavioral contributors to mortality. Psychological Bulletin, 130(6), 887–919. [DOI] [PubMed] [Google Scholar]
- Bonnet F, Irving K, Terra JL, Nony P, Berthezène F, & Moulin P (2005). Anxiety and depression are associated with unhealthy lifestyle in patients at risk of cardiovascular disease. Atherosclerosis, 178(2), 339–344. [DOI] [PubMed] [Google Scholar]
- Carnethon MR, Prineas RJ, Temprosa M, Zhang Z, Uwaifo G, Molitch ME (2006). The association among autonomic nervous system function, incident diabetes, and intervention arm in the diabetes prevention program. Diabetes Care, 29(4), 914–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, & McCrae RR (1992). Normal personality assessment in clinical practice: The NEO personality inventory. Psychological Assessment, 4(1), 5–13. [Google Scholar]
- Dermody SS, Wright AG, Cheong J, Miller KG, Muldoon MF, Flory JD, … & Manuck SB (2015). Personality Correlates of Midlife Cardiometabolic Risk: The Explanatory Role of Higher-Order Factors of the Five Factor Model. Journal of Personality, 84(6), 765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung CG (2006). Higher-order factors of the Big Five in a multi-informant sample. Journal of Personality and Social Psychology, 91(6), 1138. [DOI] [PubMed] [Google Scholar]
- Dixon-Gordon KL, Whalen DJ, Layden BK, & Chapman AL (2015). A systematic review of personality disorders and health outcomes. Canadian Psychology, 56(2), 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gabalawy R, Katz LY, & Sareen J (2010). Comorbidity and associated severity of borderline personality disorder and physical health conditions in a nationally representative sample. Psychosomatic Medicine, 72(7), 641–647. [DOI] [PubMed] [Google Scholar]
- Few LR, Grant JD, Trull TJ, Oltmanns TF, Lynskey MT, Miller JD, … Agrawal A (2016). Trait-based assessment of borderline personality disorder using the NEO five-factor inventory: Phenotypic and genetic support. Psychological Assessment, 28, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenburg FR, & Zanarini MC (2006). Obesity and obesity-related illnesses in borderline patients. Journal of Personality Disorders, 20(1), 71–80. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Marsland AL, Kuan DC, Schirda BL, Jennings JR, Sheu LK, … Manuck SB (2014). An inflammatory pathway links atherosclerotic cardiovascular disease risk to neural activity evoked by the cognitive regulation of emotion. Biological Psychiatry, 75(9), 738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greggersen W, Rudolf S, Brandt PW, Schulz E, Fassbinder E, Willenborg B, … & Schweiger U (2011). Intima-media thickness in women with borderline personality disorder. Psychosomatic medicine, 73(7), 627–632. [DOI] [PubMed] [Google Scholar]
- Greggersen W, Rudolf S, Fassbinder E, Dibbelt L, Stoeckelhuber BM, Hohagen F, …& Schweiger U (2011). Major depression, borderline personality disorder, and visceral fat content in women. European Archives of Psychiatry and Clinical Neuroscience, 261(8), 551–557. [DOI] [PubMed] [Google Scholar]
- Kahl KG, Greggersen W, Schweiger U, Cordes J, Correll CU, Frieling H, … & Moebus S (2013). Prevalence of the metabolic syndrome in patients with borderline personality disorder: Results from a cross-sectional study. European Archives of Psychiatry and Clinical Neuroscience, 263(3), 205–213. [DOI] [PubMed] [Google Scholar]
- Kinnunen M, Kokkonen M, Kaprio J, & Pulkkinen L (2005). The associations of emotion regulation and dysregulation with the metabolic syndrome factor. Journal of Psychosomatic Research, 58(6), 513–521. [DOI] [PubMed] [Google Scholar]
- Kop WJ, Synowksi SJ, & Gottlieb SS (2011). Depression in heart failure: Biobehavioral mechanisms. Heart Failure Clinics, 7(1), 23–38. [DOI] [PubMed] [Google Scholar]
- Lee HB, Bienvenu O. Joseph, Cho Seong-Jin, Ramsey, Bandeen-Roche K, Eaton WW, & Nestadt Gerald, (2011). Personality disorders and traits as predictors of incident cardiovascular disease: Findings from the 23-year follow-up of the Baltimore ECA study. Psychosomatics, 51(4), 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JC, Wallace ML, Fournier JC, Rucci P, & Frank E (2012). The role of personality pathology in depression treatment outcome with psychotherapy and pharmacotherapy. Journal of Consulting and Clinical Psychology, 80(5), 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S, Wong ND, Franklin SS, Kamath TV, Gilbert JL, Pio JR, & Williams GR (2004). Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation, 110, 1245–1250. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Phillips J, Gianaros PJ, Flory JD, & Muldoon MF (2010). Subjective socioeconomic status and presence of the metabolic syndrome in midlife community volunteers. Psychosomatic medicine, 72(1), 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, McCaffery JM, Muldoon MF, & Manuck SB (2010). Systemic inflammation and the metabolic syndrome among middle-aged community volunteers. Metabolism, 59(12), 1801–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffery JM, Marsland AL, Strohacker K, Muldoon MF, & Manuck SB (2012). Factor structure underlying components of allostatic load. Plos One, 7(10), e47246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran P, Stewart R, Brugha T, Bebbington P, Bhugra D, Jenkins R, & Coid JW (2007). Personality disorder and cardiovascular disease: results from a national household survey. The Journal of Clinical Psychiatry, 68(1), 69–74. [DOI] [PubMed] [Google Scholar]
- Muldoon MF, Nazzaro P, Sutton-Tyrrell K, & Manuck SB (2000). White-coat hypertension and carotid artery atherosclerosis: a matching study. Archives of internal medicine, 160(10), 1507–1512. [DOI] [PubMed] [Google Scholar]
- Pan A, Sun Q, Okereke OI, Rexrode KM, & Hu FB (2011). Bidirectional association between depression and metabolic syndrome: a meta-analysis and systematic review. Journal of the American Medical Association, 306, 1241–1249.21934057 [Google Scholar]
- Powers AD, & Oltmanns TF (2013). Borderline personality pathology and chronic health problems in later adulthood: The mediating role of obesity. Personality Disorders: Theory, Research, and Treatment, 4(2), 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk SE, Berk M, Chanen AM, Koivumaa-Honkanen H, Brennan-Olsen SL, Pasco JA, & Williams LJ (2016). Population prevalence of personality disorder and associations with physical health comorbidities and health care service utilization: A review. Personality Disorders: Theory, Research, and Treatment, 7(2), 136. [DOI] [PubMed] [Google Scholar]
- Rugulies R (2002). Depression as a predictor for coronary heart disease. A review and meta-analysis. American Journal of Preventive Medicine, 23, 51–61. [DOI] [PubMed] [Google Scholar]
- Sansone RA, Wiederman MW, & Monteith D (2001). Obesity, borderline personality symptomatology, and body image among women in a psychiatric outpatient setting. International Journal of Eating Disorders, 29(1), 76–79. [DOI] [PubMed] [Google Scholar]
- Schiepers OJG, Wichers MC, & Maes M (2005). Cytokines and major depression. Progress in Neuropsychopharmacology & Biological Psychiatry, 29(2), 201–217. [DOI] [PubMed] [Google Scholar]
- Shafer AB (2006). Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. Journal of Clinical Psychology, 62(1), 123–146. [DOI] [PubMed] [Google Scholar]
- Shen B, Todaro JF, Niaura R, McCaffery JM, Zhang J, Spiro III A, & Ward KD (2003). Are metabolic risk factors one unified syndrome? modeling the structure of the metabolic syndrome X. American Journal of Epidemiology, 157(8), 701–711. [DOI] [PubMed] [Google Scholar]
- Strine TW, Mokdad AH, Balluz LS, Gonzalez O, Crider R, Berry JT, Kroenke K (2005). Depression and anxiety in the United States: Finding from the 2006 Behavioral Risk Factor Surveillance System. Psychiatric Services, 59(12), 1383–1390. [DOI] [PubMed] [Google Scholar]
- Tomko RL, Trull TJ, Wood PK, & Sher KJ (2014). Characteristics of borderline personality disorder in a community sample: Comorbidity, treatment utilization, and general functioning. Journal of Personality Disorders, 28(5), 734–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trull TJ, Freeman LK, Vebares TJ, Choate AM, Helle AC, & Wycoff AM (2018). Borderline personality disorder and substance use disorders: An updatedreview. Borderline Personality Disorder and Emotion Dysregulation, 5(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtorta NK, Kanaan M, Gilbody S, Ronzi S, & Hanratty B (2016). Loneliness and social isolation as risk factors for coronary heart disease and stroke: Systematic review and meta-analysis of longitudinal observational studies. Heart, 102(13), 1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancampfort D, Correll CU, Wampers M, Sienaert P, Mitchell AJ, De Herdt A, Probst M, Scheewe TW, & De Hert M (2014). Metabolic syndrome and metabolic abnormalities in patients with major depressive disorder: a meta-analysis of prevalences and moderating variables. Psychological Medicine, 44, 2017–2028. [DOI] [PubMed] [Google Scholar]