Abstract
Background:
One approach to decreasing the cesarean delivery rate in the USA is to increase the availability of birth attendants, including certified nurse midwives (CNMs), who offer trial of labor after cesarean (TOLAC). We examined associations between provider type and mode of birth for women attempting vaginal birth after cesarean (VBAC).
Methods:
We performed a retrospective cohort study at a United States academic medical center using prospectively collected data (2005–2012). We included healthy women with term singleton vertex pregnancies after one or two prior cesareans who were managed by obstetricians or CNMs. We assessed unplanned cesarean delivery by provider type using univariate and logistic regression and examined labor interventions and predicted VBAC success.
Results:
Overall VBAC success was 88% for 502 included patients. Unplanned cesarean rates were similar by provider type. Black race, no prior VBAC, recurring clinical indication for cesarean, labor augmentation/induction, and any Pitocin use were associated with increased unplanned cesarean. Higher parity and early-term gestational age at delivery were associated with decreased unplanned cesarean. Postpartum hemorrhage and composite maternal morbidity were increased with unplanned cesarean, but there was no difference in neonatal outcome by mode of delivery or provider type. Obstetricians had slightly higher composite adverse maternal outcomes. Nomogram-predicted VBAC success but not provider type was associated with unplanned cesarean.
Conclusions:
Unplanned cesarean was similar for patients attempting labor after cesarean managed by midwives or obstetricians. Increasing the number of CNMs who manage TOLAC may help decrease the high rate of cesareans.
Keywords: obstetrician, midwifery, failed TOLAC, predicted VBAC success, cesarean section
INTRODUCTION
The cesarean delivery rate in the United States has remained at 31% or higher for over a decade.(1) The first cesarean accounts for the majority of this high rate, but repeat cesarean is also a major contributor and confers increased maternal morbidity and neonatal complications.(2, 3) Decreasing the first cesarean has become a major public health goal, but improving the availability and success of trial of labor after cesarean (TOLAC) is another approach to increasing vaginal delivery rates. Only 2.9% of all deliveries from 2016–2018 were attempted TOLACs, and among women with a prior cesarean TOLAC rates have fallen from ~50% in 1995 to ~18% in 2016–2018.(4, 5) This decrease is largely due to concerns for safety, adequate resources, and available providers.(6–9) In 2012, only 57% of hospitals in California and 41% of hospitals in New Mexico permitted TOLAC.(8, 10) Furthermore, in one survey, only one-half of private practitioners in North Texas offered their patients TOLAC.(6) Increasing the pool of providers who offer labor after cesarean might be an effective approach to increase vaginal delivery opportunities.
Successful VBAC occurs in 60–80% of women attempting TOLAC overall, but there are scant data on TOLAC success by provider type.(11–13) One study of birthing centers found certified nurse midwives (CNMs) achieved high TOLAC success.(14) The American College of Obstetricians & Gynecologists (ACOG) suggests that TOLAC should only be undertaken at “facilities that can provide cesarean delivery for situations that are immediate threats to the life of the woman or fetus.”(15) The capability for immediate cesarean delivery is not available at all hospitals or birthing centers, and, therefore, those sites may choose not to offer TOLAC. Although the absolute risk of uterine rupture with labor after cesarean is less than 1%, this remains a significant concern that can be well addressed in a collaborative care model including immediately available surgeons.(16, 17) Perinatal morbidity is higher in TOLAC (0.13%) compared to elective repeat cesarean (0.05%), but the absolute risks are very low.(16) Additionally, beyond the immediate surgical and postoperative risks, cesarean delivery increases lifelong risks for the offspring, with higher rates of asthma, diabetes, and childhood obesity compared to infants delivered vaginally.(18, 19) Successful VBAC reduces maternal risk for transfusion, unplanned hysterectomy, and intensive care unit admission.(13) Despite the small but potentially catastrophic risks of TOLAC, it remains cost-effective and provides improved long-term health outcomes compared to scheduled repeat cesarean.(20)
Data suggest that CNMs may have lower overall unplanned cesarean rates compared to obstetricians managing low-risk pregnancies,(21–23) but the rate of cesarean and the risks by provider type specifically for labor after cesarean are not known. We hypothesized that CNM management of low-risk TOLAC patients increases vaginal delivery success without additional morbidity. To study this, we compared cesarean for TOLAC patients managed by CNMs and obstetricians and evaluated maternal and neonatal outcomes.
METHODS
We performed a retrospective cohort study using the University of Colorado Perinatal Database, which includes all women delivered at the University of Colorado Hospital tertiary care academic medical center between October 1, 2005 through December 31, 2012. Trained research assistants abstracted delivery data prior to discharge from the hospital. In order to confirm database accuracy, we verified study variables from the original medical record for 26% of the cohort and found over 98% agreement between University of Colorado Perinatal Database variables and the medical patient record.(21) The Colorado Multiple Institutional Review Board approved this study (COMIRB protocol #16–1786).
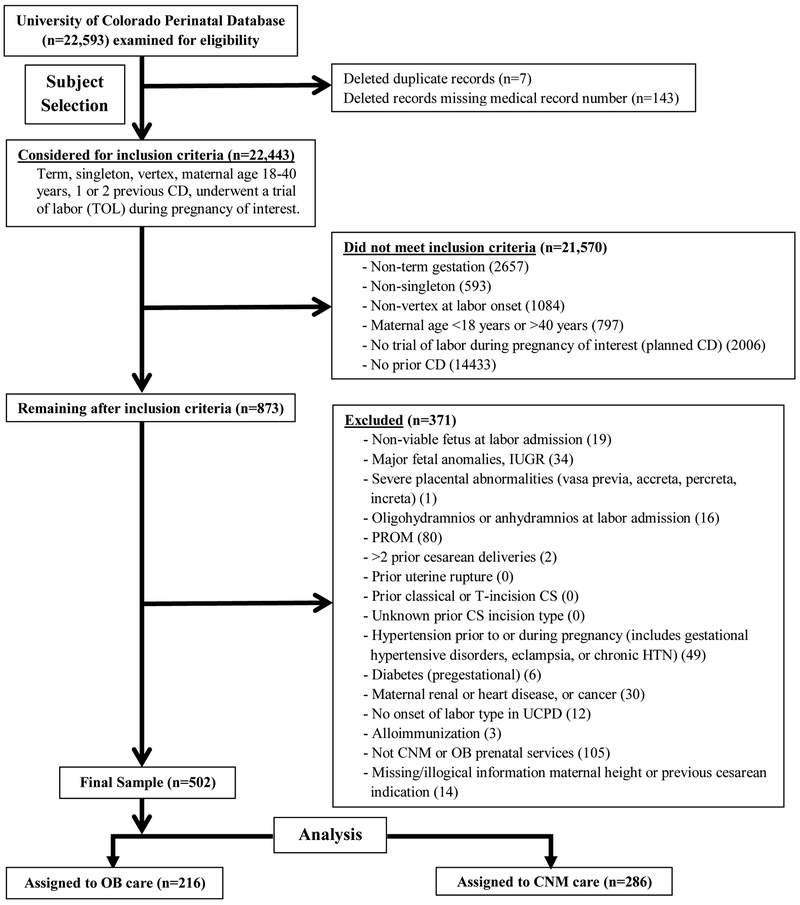
For our analysis, we included low-risk deliveries with these characteristics: singleton, term gestation (37 0/7 through 41 6/7 weeks with estimated date of delivery determined by last menstrual period [LMP] and/or ultrasound), vertex presentation, maternal age 18 through 40 years with one or two previous cesarean sections, and attempting labor. Subjects were excluded for any of the following: high-risk pregnancy (not typically cared for by CNMs), missing medical record number, duplicate record, or cared for by a family practice group. High-risk pregnancies were defined as non-viable fetus at labor admission, major fetal anomalies, intrauterine growth restriction (IUGR), severe placental abnormalities, oligo- or an-hydramnios, prelabor rupture of membranes (PROM), prior uterine rupture/classical cesarean/’T’-incision, unknown prior cesarean incision type, hypertension, pregestational diabetes, maternal renal or heart disease, maternal cancer, or alloimmunization. In total, 502 women made up our cohort (Figure 1). Race and ethnicity were self-reported. We calculated pre-pregnancy body mass index (BMI) using height and weight before pregnancy or in the first trimester, whereas BMI at delivery was the BMI calculated at hospital admission.(24) From these data, we calculated maternal gestational weight gain, which was classified as appropriate/below or excessive based on the Institute of Medicine’s recommendations.(25) We obtained the mothers’ prior TOLAC attempts to assess outcomes by prior VBAC history (e.g. previous VBAC, failed TOLAC, or no attempt) and determined whether the indication for the prior cesarean was recurring (e.g. abnormal labor progress, failed induction of labor, failed prior VBAC) or not (e.g. non-reassuring fetal status). Our a priori sample size calculation required at least 97 patients for each provider type using p<0.05 and 80% power with an overall unplanned cesarean rate of 50% +/−5% and 20% difference in unplanned cesarean between provider groups.
Figure 1.
Subject selection flow diagram, Denver, Colorado, United States, 2005–2012.
In a review of 348 randomly selected charts, provider type was confirmed by comparison to a separate CNM birth data set; we found no errors in provider assignment or mode of birth. Obstetric providers were faculty members in general obstetrics-gynecology or maternal fetal medicine. CNMs included both faculty and private midwifery practices (i.e. all obstetricians and all midwives delivering at University of Colorado Hospital were included). Private CNM patients comprised 30% of the CNM group. In addition, some low-risk women receive care in community clinics based on proximity to the patient’s home. These clinics are staffed by either obstetricians or midwives, and the delivering provider type was the same. Although obstetricians care for all women with complicated pregnancies, we limited our cohort to healthy, low-risk women. Hospital and private CNM practices use common nursing staff and have similar institutional privileges. The CNMs practice independently unless complications arise for which they request obstetric consultation. Although operative vaginal births and cesarean delivery require transfer of care from CNM to obstetrician, we analyzed by original provider type only (i.e. intention-to-manage analysis). Both midwifery and obstetric practices incorporate trainees.
Gestational age at birth and neonatal birthweight were obtained from the birth summary. Mode of delivery outcomes were vaginal delivery, operative vaginal delivery (forceps or vacuum), and unplanned cesarean. Reasons for unplanned cesarean were primary indication of failure to dilate/descend, labor dystocia, or fetal intolerance of labor/non-reassuring fetal heart tracing. We also assessed neonatal adverse outcomes (surfactant administration, APGAR score less than seven at five minutes, neonatal intensive care unit admission in the initial 24 hours, respiratory distress syndrome diagnosis, shoulder dystocia, neonatal death, and neonatal resuscitation, including blow-by oxygen requirement, positive pressure ventilation/CPAP, intubation, and CPR/resuscitative measures) and maternal adverse outcomes (intrapartum fever, postpartum hemorrhage, maternal death, episiotomy, third or fourth degree perineal laceration, cesarean or postpartum hysterectomy). We examined a maternal morbidity composite outcome of chorioamnionitis, hysterectomy, or postpartum hemorrhage and a neonatal morbidity composite of APGAR score <7 at five minutes, surfactant administration, neonatal intensive care unit admission, or intubation. An overall composite adverse outcome was positive if any maternal or neonatal adverse outcome was recorded.
For our entire cohort, we calculated proportions and unadjusted odds ratios for provider type and delivery mode by maternal, pregnancy, and neonatal characteristics and by labor complications, interventions, and outcomes. For characteristics with more than two categories, p-values for the overall chi-square are reported. We then developed a multivariable logistic regression model for unplanned cesarean, adjusting for factors that were significantly different in the unadjusted analysis (maternal race, VBAC history, labor augmentation or induction, any Pitocin [synthetic oxytocin] use, neuraxial analgesia use, and gestational age at delivery). Only factors that remained significant in regression analysis were retained.
We calculated the probability of VBAC success using the nomogram from Grobman et al. (2007). The Grobman score is the predicted percent VBAC success based on readily available clinical data including maternal age, height, weight, BMI, race/ethnicity, prior vaginal delivery, vaginal delivery since last cesarean, and arrest of dilation or descent as the indication for the previous cesarean.(26) We used the Grobman score to evaluate whether predicted VBAC success influenced the difference in unplanned cesarean by provider type. Using receiver operator characteristics analysis, we classified scores of 0.65 or greater as high probability for VBAC success. In a sensitivity analysis, we analyzed matched case-control subgroups. In a second sensitivity analysis, we adjusted provider type comparisons for unplanned cesarean by nomogram-predicted VBAC success. P-values <0.05 were considered significant. We used SAS 9.4 software for data analysis and GraphPad Prism 6 for figure preparation.
RESULTS
Cohort Composition and Demographics by Provider Type
We examined 22,593 pregnancies in the perinatal database for eligibility. Of those, 873 met inclusion criteria, and 502 remained after all exclusions (Figure 1). Midwives managed 57%, and obstetricians managed 43% of these women, and the overall predicted VBAC success (Grobman score) was not different by provider type. The cohort was mostly normal-weight Hispanic and white women between 25–34 years old (Table 1). Maternal demographics were similar by provider type except that obstetric patients were more frequently early-term gestational age at delivery (37 0/7 through 38 6/7 weeks), whereas CNM patients were more likely to be full-term and late-term (39 0/7 to 42 weeks). The rate of successful VBAC compared to all vaginal births in the entire database (not limited to low-risk patients) was 6.8% (1,115/16,360) which is similar to the 4.3% VBAC rate over the past three years at our institution (July 2015 through June 2018); this suggests that the perinatal database accurately captured most TOLAC attempts.
Table 1:
Patient demographics for the overall cohort and by provider type, Denver, Colorado, United States, 2005–2012.
| Characteristic | Overall1 N=502, No. (%) | Certified Nurse Midwife N=286, No. (%) | Obstetrician N=216, No. (%) | P value |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Maternal age (years) | ||||
| 18–24 | 116 (23.1) | 63 (22.0) | 53 (24.5) | 0.543 |
| 25–34 | 318 (63.4) | 187 (65.4) | 131 (60.7) | |
| 35–40 | 68 (13.6) | 36 (12.6) | 32 (14.8) | |
| Pre-pregnancy BMI2,3 | ||||
| <18.5 | 10 (2.1) | 4 (1.5) | 6 (2.9) | 0.226 |
| 18.5–29.9 | 371 (77.1) | 220 (79.7) | 151 (73.7) | |
| ≥30 | 100 (20.8) | 52 (18.8) | 48 (23.4) | |
| BMI at delivery2 | ||||
| 18.5–29.9 | 240 (47.8) | 136 (47.6) | 104 (48.1) | 0.895 |
| ≥30 | 262 (52.2) | 150 (52.5) | 112 (51.9) | |
| Gestational weight gain3 | ||||
| Appropriate/Below | 274 (57.0) | 159 (57.6) | 115 (56.1) | 0.741 |
| Excessive | 207 (43.0) | 117 (42.4) | 90 (43.9) | |
| Race/Ethnicity | ||||
| Hispanic | 251 (50.0) | 146 (51.1) | 105 (48.6) | 0.222 |
| Black | 59 (11.8) | 32 (11.2) | 27 (12.5) | |
| Non-Hispanic white | 146 (29.1) | 88 (30.8) | 58 (26.9) | |
| Other | 46 (9.2) | 20 (7.0) | 26 (12.0) | |
| Marital status(married or partnered) | 371 (73.9) | 205 (71.7) | 166 (76.9) | 0.191 |
| Maternal height (<60 inches) | 31 (6.2) | 21 (7.3) | 10 (4.6) | 0.211 |
| Smoked during pregnancy | 35 (7.0) | 21 (7.3) | 14 (6.5) | 0.708 |
| Gestational diabetes | 28 (5.6) | 11 (3.9) | 17 (7.9) | 0.052 |
| Obstetric Characteristics | ||||
| Gravidity | ||||
| 2 | 177 (35.3) | 100 (35.0) | 77 (35.7) | 0.874 |
| >2 | 325 (64.7) | 186 (65.0) | 139 (64.4) | |
| Parity | ||||
| 1 | 260 (51.8) | 153 (53.5) | 107 (49.5) | 0.379 |
| ≥2 | 242 (48.2) | 133 (46.5) | 109 (50.5) | |
| Successful previous VBAC4 | ||||
| Yes | 135 (26.9) | 80 (28.1) | 55 (25.5) | 0.545 |
| No | 365 (72.9) | 204 (71.6) | 161 (74.5) | |
| Uncertain | 1 (0.2) | 1 (0.4) | 0 | |
| Failed a previous TOLAC | 3 (0.6) | 2 (0.7) | 1 (0.5) | 0.734 |
| Recurring indication for repeated cesarean | 195 (38.8) | 121 (42.3) | 74 (34.3) | 0.067 |
| Grobman score (probability)5 | ||||
| <0.65 (low) | 168 (34.9) | 106 (38.4) | 62 (30.2) | 0.067 |
| ≥0.65 (high) | 313 (65.1) | 170 (61.6) | 143 (69.8) | |
| Fetal characteristics | ||||
| Gestational age at delivery(weeks days) | ||||
| 37 0/7 – 38 6/7 | 130 (25.9) | 60 (21.0) | 70 (32.4) | 0.010 |
| 39 0/7 – 40 6/7 | 299 (59.6) | 178 (62.2) | 121 (56.0) | |
| 41 0/7 – 41 6/7 | 73 (14.5) | 48 (16.8) | 25 (11.6) | |
| Birthweight (g) | ||||
| <2,500 | 5 (1.0) | 1 (0.4) | 4 (1.9) | 0.171 |
| 2500–4200 | 482 (96.0) | 278 (97.2) | 204 (94.4) | |
| >4200 | 15 (3.0) | 7 (2.5) | 8 (3.7) | |
| Clinical suspicion of macrosomia present6 | 12 (2.4) | 7 (2.5) | 5 (2.3) | 0.923 |
| Labor interventions | ||||
| Status of onset of labor | ||||
| Spontaneous | 303 (60.4) | 186 (65.0) | 117 (54.2) | 0.027 |
| Augmentation | 155 (30.9) | 81 (28.3) | 74 (34.3) | |
| Induction | 44 (8.8) | 19 (6.6) | 25 (11.6) | |
| Artificial rupture of membranes | 263 (52.4) | 136 (47.6) | 127 (58.8) | 0.013 |
| Episiotomy performed | 10 (2.0) | 5 (1.8) | 5 (2.3) | 0.653 |
| Received epidural | 244 (48.6) | 124 (43.4) | 120 (55.6) | 0.007 |
| Prostaglandin use | 9 (1.8) | 3 (1.1) | 6 (2.8) | 0.148 |
| Pitocin | 189 (37.7) | 94 (32.9) | 95 (44.0) | 0.011 |
, N for each column may vary due to missing variables for each factor.
, Mean BMI (kg/m2)(24)
, Pre-pregnancy weight, height, or weight at delivery was incomplete for twenty-one patients based on chart review.
, Prior VBAC percentages were calculated by dividing the number of ‘yes’, ‘no’, or ‘uncertain’ by the sum of the entire “successful previous VBAC” category for that provider type/column.
, Unable to calculate the Grobman probability score if components were missing. Optimal 0.65 cutoff was determined by receiver operator characteristics curve analysis.
, Based on documented clinical assessment not uniform estimated fetal weight definition.
Factors Associated with Delivery Mode
Maternal factors associated with increased unplanned cesarean included black race (OR 2.45, 95% CI 1.17–5.11) and no prior successful VBAC (OR 5.67, 95% CI 2.01–15.97). Protective factors decreasing the risk of unplanned cesarean included increased gravidity >2 (OR 0.46, 95% CI 0.27–0.80), increased parity ≥2 (OR 0.33, 95% CI 0.18–0.62), and early term gestational age 37 0/7 – 38 6/7 weeks (OR 0.40, 95% CI 0.17–0.93). Some of these risks (black race and no prior VBAC) and protections (gestational age 37 0/7–38 6/7 weeks) for unplanned cesarean are consistent with prior findings.(26–28) Also similar to prior reports,(27, 28) labor interventions associated with increased risk of unplanned cesarean included labor augmentation (OR 5.16, 95% CI 2.65–10.04), labor induction (OR 8.66, 95% CI 3.73–20.07), and any Pitocin use (OR 5.30, 95% CI 2.89–9.75; Table 2).
Table 2:
Proportion of unplanned cesareans by provider type and unadjusted odds ratios for unplanned cesarean delivery by maternal and pregnancy characteristics, intrapartum interventions, and labor complications, Denver, Colorado, United States, 2005–2012.
| Characteristic | Unplanned Cesarean1 N=58,No.(%) | Successful VBAC N=444, No. (%) | Unadjusted Odds Ratio (95% CI) | P value |
|---|---|---|---|---|
| Delivery mode outcome2 | ||||
| Overall (N=502) | 58 (11.6) | 0.404 | ||
| Midwife (N=286) | 36 (12.6) | |||
| Obstetrician (N=216) | 22 (10.2) | |||
| Maternal characteristics | ||||
| Maternal age (years) | ||||
| 18–24 | 16 (27.6) | 100 (22.5) | 0.93 (0.40–2.18) | 0.38 |
| 25–34 | 32 (55.2) | 286 (64.4) | 0.65 (0.30–1.39) | |
| 35–40 | 10 (17.2) | 58 (13.1) | Reference | |
| Pre-pregnancy BMI3,4 | ||||
| <18.5 | 1 (1.8) | 9 (2.1) | 0.95 (0.12–7.67) | 0.225 |
| 18.5–29.9 | 39 (68.4) | 332 (78.3) | Reference | |
| ≥30 | 17 (29.8) | 83 (19.6) | 1.74 (0.94–3.24) | |
| BMI at delivery3,4 | ||||
| 18.5–29.9 | 25 (43.1) | 215 (48.4) | Reference | 0.486 |
| ≥30 | 33 (56.9) | 229 (51.6) | 1.24 (0.71–2.15) | |
| Gestational weight gain4 | ||||
| Appropriate/Below | 29 (50.9) | 245 (57.8) | Reference | 0.393 |
| Excessive | 28 (49.1) | 179 (42.2) | 1.32 (0.76–2.30) | |
| Race/Ethnicity | ||||
| Hispanic | 26 (44.8) | 225 (50.7) | Reference | <0.05 |
| Black | 13 (22.4) | 46 (10.4) | 2.45 (1.17–5.11) | |
| Non-Hispanic white | 13 (22.4) | 133 (30.0) | 0.85 (0.42–1.70) | |
| Other | 6 (10.3) | 40 (9.0) | 1.30 (0.50–3.35) | |
| Marital status (married or partnered) | 37 (63.8) | 334 (75.2) | 0.58 (0.33–1.03) | 0.079 |
| Maternal height (<60 inches) | 5 (8.6) | 26 (5.9) | 1.52 (0.56–4.12) | 0.561 |
| Smoked during pregnancy | 7 (12.1) | 28 (6.3) | 2.04 (0.85–4.91) | 0.163 |
| Gestational diabetes | 4 (6.9) | 24 (5.4) | 1.30 (0.43–3.88) | 0.759 |
| Obstetrical Characteristics | ||||
| Gravidity | ||||
| 2 | 30 (51.7) | 147 (33.1) | Reference | 0.006 |
| >2 | 28 (48.3) | 297 (66.9) | 0.46 (0.27–0.80) | |
| Parity | ||||
| 1 | 43 (74.1) | 217 (48.9) | Reference | <.001 |
| ≥2 | 15 (25.9) | 227 (51.1) | 0.33 (0.18–0.62) | |
| Successful previousVBAC | ||||
| Yes | 4 (6.9) | 131 (29.6) | Reference | <.001 |
| No | 54 (93.1) | 312 (70.4) | 5.67 (2.01–15.97) | |
| Failed a previous TOLAC | 1 (1.7) | 2 (0.5) | 3.88 (0.35–43.44) | 0.309 |
| Recurring indicationfor cesarean | 33 (56.9) | 162 (36.5) | 2.30 (1.32–4.00) | 0.004 |
| Grobman score (probability)5 | ||||
| <0.65 (low) | 37 (64.9) | 131 (30.9) | 4.14 (2.31–7.40) | <.001 |
| ≥0.65 (high) | 20 (35.1) | 293 (69.1) | Reference | |
| Fetal characteristics | ||||
| Gestational age at delivery (weeks days) | ||||
| 37 0/7 – 38 6/7 | 7 (12.1) | 123 (27.7) | 0.40 (0.17–0.93) | 0.01 |
| 39 0/7 – 40 6/7 | 37 (63.8) | 262 (59.0) | Reference | |
| 41 0/7 – 41 6/7 | 14 (24.1) | 59 (13.3) | 1.68 (0.85–3.31) | |
| Clinical suspicion of macrosomia present6 | 2 (3.5) | 10 (2.3) | 1.55 (0.33–7.26) | 0.638 |
| Intrapartum interventions | ||||
| Status of onset of labor | ||||
| Spontaneous | 14 (24.1) | 289 (65.1) | Reference | <.001 |
| Augmentation | 31 (53.5) | 124 (27.9) | 5.16 (2.65–10.04) | |
| Induction | 13 (22.4) | 31 (7.0) | 8.66 (3.73–20.07) | |
| Artificial rupture of embranes | 30 (51.7) | 233 (52.5) | 0.97 (0.56–1.68) | >.99 |
| Episiotomy performed | NA | 10 (2.3) | NA | 0.387 |
| Received epidural | 34 (58.6) | 210 (47.3) | 1.58 (0.91–2.75) | 0.124 |
| Prostaglandin use | 2 (3.5) | 7 (1.6) | 2.23 (0.45–11.00) | 0.607 |
| Pitocin | 42 (72.4) | 147 (33.1) | 5.30 (2.89–9.75) | <.001 |
NA = not applicable
, N for each column may vary due to missing variables for each factor.
, Control (reference) group was care with CNM. Delivery mode for our model outcome was unplanned cesarean. P value for only this characteristic applies to comparison of successful VBAC vs unplanned cesarean by provider type.
, Mean BMI (kg/m2)(24)
, Pre-pregnancy weight, height, or weight at delivery was incomplete for twenty-one patients based on chart review.
, Unable to calculate the Grobman probability score if components were missing. Optimal 0.65 cutoff was determined by receiver operator characteristics analysis.
, Based on documented clinical assessment not uniform estimated fetal weight definition.
Cesarean Delivery and Labor Management Associated with Provider Type
The absolute difference in unplanned cesarean rate by provider type was 2.4% (a relative decrease of 19% for obstetricians) which was not significant. The incidence of transfer from CNM to obstetrician care was 16.8%. There was no significant association between unplanned cesarean and provider type in either unadjusted or adjusted analyses (unadjusted OR 0.78, 95% CI 0.45–1.37; adjusted OR 0.60, 95% CI 0.32–1.12; Table 3 and Supplemental Table 1 published online only). To compare provider types by predicted VBAC success, we examined predicted versus actual VBAC rates. Although low probability of VBAC success (score <0.65) was associated with unplanned cesarean (OR 4.14, 95% CI 2.31–7.40), there was no difference by provider type across all predicted VBAC success scores (Table 1 and Figure 2). Overall, the rate of successful VBAC for patients attempting TOLAC was 88% (i.e., the number of successful VBACs for all attempted TOLACs). Over time, the rate of unsuccessful TOLAC increased about two-fold in the final two years of data collection, decreasing the VBAC success to ~80% (Supplemental table 2 published online only). In a sensitivity analysis among matched cases and controls, the lack of significant difference by provider type persisted in both unadjusted or adjusted analysis (Supplemental Tables 3 and 4 published online only). Patients managed by obstetricians were more likely to undergo intrapartum interventions, such as labor induction and augmentation, artificial rupture of membranes, epidural analgesia, and use of Pitocin (Table 1).
Table 3:
Unadjusted or adjusted odds ratios (with regression model) for association of unplanned cesarean delivery with obstetrician provider type, Denver, Colorado, United States, 2005–2012.
| Independent Variables | Unadjusted OR & 95% CI for obstetric provider group | Final Adjusted OR & 95% CI1 |
|---|---|---|
| Provider Type | ||
| Midwife | Reference | |
| Obstetrician | 0.78 (0.45–1.37) | 0.60 (0.32–1.12) |
| Maternal characteristics | ||
| Race | ||
| Non-Hispanic white | Reference | |
| Hispanic | 1.34 (0.63–2.85) | |
| Black | 4.18 (1.65–10.6) | |
| Other | 1.96 (0.64–6.03) | |
| Obstetric history | ||
| History of any prior VBAC | Reference | |
| Most recent previous delivery was cesarean | 4.99 (1.66–15) | |
| No prior VBAC | 3.75 (1.08–13) | |
| Recurring maternal indication for cesarean | ||
| Yes | Reference | |
| None | 0.43 (0.23–0.79) | |
| Labor onset & interventions | ||
| Induction | Reference | |
| Spontaneous onset of labor | 0.09 (0.04–0.22) |
Bolding denotes statistical significance
, Adjusted model includes race-ethnicity, VBAC history (prior successful VBAC, cesarean as most recent delivery, no prior successful VBAC), non-recurring indication for cesarean, and spontaneous onset of labor.
Figure 2:
Proportions and 95% CI of unplanned cesarean for predicted VBAC success scores by obstetrician or midwife provider type, Denver, Colorado, United States, 2005–2012. Subjects were divided by provider type and then into quintiles by predicted Grobman VBAC success score. The 95% confidence interval for proportion of unplanned cesarean within each quintile is graphed versus the predicted VBAC success. Line of agreement shows perfect match between predicted and actual cesarean rates.
Morbidity Associated with Delivery Mode and Provider Type
We evaluated the association of provider type and delivery mode with maternal and neonatal morbidity (Table 4). Postpartum hemorrhage (OR 2.51, 95% CI 1.31–4.83), chorioamnionitis (OR 4.28, 95% CI 1.74–10.51), maternal composite morbidity (OR 3.14, 95% CI 1.73–5.69), and combined-composite (maternal or neonatal) morbidity (OR 2.78, 95% CI 1.56–4.94) were associated with unplanned cesarean. Maternal composite morbidity was significantly higher for obstetric provider type compared to CNM (p=0.045), due to increased chorioamnionitis and third- and fourth-degree perineal lacerations. The neonatal composite and combined composite were not significantly different by provider type.
Table 4:
Adverse outcomes by provider type and unadjusted odds ratios for adverse outcomes by mode of delivery, Denver, Colorado, United States, 2005–2012.
| Adverse Outcome | Overall N=502, No. (%) | Certified Nurse Midwife N=286, No. (%) | Obstetrician N=216, No. (%) | P value | Unplanned Cesarean N=58, No. (%) | Successful VBAC N=444, No (%) | Delivery Mode Odds Ratio (95% CI) | P value |
| Labor complications | ||||||||
| Shoulder dystocia | 18 (3.6) | 9 (3.2) | 9 (4.2) | 0.63 | NA | 18 (4.1) | - | 0.151 |
| Maternal | ||||||||
| Chorioamnionitis | 24 (4.8) | 10 (3.5) | 14 (6.5) | 0.141 | 8 (13.8) | 16 (3.6) | 4.28 (1.74–10.51) | 0.003 |
| Postpartum hemorrhage | 69 (13.8) | 34 (11.9) | 35 (16.2) | 0.191 | 15 (25.9) | 54 (12.2) | 2.51 (1.31–4.83) | 0.007 |
| Hysterectomy | 1 (0.2) | 1 (0.4) | 0 | >.99 | 1 (1.7) | 0 | - | 0.116 |
| Perineal laceration, 3rd or 4th degree | 11 (2.2) | 3 (1.1) | 8 (3.7) | 0.063 | NA | 11 (2.5) | - | 0.379 |
| Neonatal | ||||||||
| APGAR score at 5minutes | ||||||||
| <7 | 2 (0.4) | 2 (0.7) | 0 | 0.509 | 2 (3.5) | 0 | - | 0.013 |
| ≥7 | 500 (99.6) | 284 (99.3) | 216 (100.0) | 56 (96.6) | 444 (100.0) | - | ||
| Surfactant given | 1 (0.2) | 1 (0.4) | 0 | >.99 | 0 | 1 (0.2) | - | >.99 |
| Neonatal intensive care unit admission | 22 (4.4) | 16 (5.6) | 6 (2.8) | 0.185 | 2 (3.5) | 20 (4.5) | 0.76 (0.17–3.33) | 0.764 |
| Intubation | 5 (1.0) | 2 (0.7) | 3 (1.4) | 0.656 | 1 (1.7) | 4 (0.9) | 1.93 (0.21–17.53) | >.99 |
| Composite | ||||||||
| Maternal1 | 89 (17.7) | 42 (14.7) | 47 (21.8) | 0.045 | 21 (36.2) | 68 (15.3) | 3.14 (1.73–5.69) | <.001 |
| Neonate2 | 27 (5.4) | 18 (6.3) | 9 (4.2) | 0.325 | 4 (6.9) | 23 (5.2) | 1.36 (0.45–4.07) | 0.757 |
| Overall3 | 108 (21.5) | 57 (19.9) | 51 (23.6) | 0.326 | 23 (39.7) | 85 (19.1) | 2.78 (1.56–4.94) | <.001 |
NA = not applicable
, Maternal composite combined maternal outcomes of chorioamnionitis, hysterectomy, postpartum hemorrhage, and maternal death, the last of which was not observed.
, Neonatal composite definition included APGAR score <7 at 5 min, surfactant administration, neonatal intensive care unit admission, and intubation. It also included positive pressure ventilation/CPAP, respiratory distress syndrome, CPR, and neonatal death, but none were observed.
, Positive if any adverse maternal or neonatal outcome was positive.
DISCUSSION
We did not detect a significant difference in unplanned cesarean rates for low-risk TOLAC patients cared for by midwives or obstetricians in our intention-to-manage analysis. This was true after adjusting for variables that were significantly different by provider type or mode of delivery (race, prior VBAC, recurring maternal indication for cesarean, most recent delivery mode, and spontaneous labor). We identified associations with unplanned cesarean in this cohort similar to risks reported previously for unsuccessful TOLAC (black race, recurring maternal indication for cesarean, induction of labor, labor augmentation, and Pitocin use).(27, 28) Adjusted analysis also showed risks for unplanned cesarean in non-Hispanic black women that were consistent with prior findings.(29) Factors that increased VBAC success were also identified (successful prior VBAC, spontaneous labor, and high Grobman score).(26–28) Obstetricians more frequently utilized labor induction, epidurals, and Pitocin.(20, 21, 30) Morbidity by provider type was not significant, except that composite maternal morbidity was slightly higher for patients managed by obstetricians. Several adverse outcomes were associated with unplanned cesarean, including chorioamnionitis and postpartum hemorrhage.
Identifying new health care delivery systems and management models may be an effective way to increase vaginal delivery rates in the USA. Nationally, CNMs attend 13.4% of vaginal births and 9.1% of all births.(31) Some reports have shown lower cesarean rates overall for patients managed by midwives,(21, 22, 32) but we found no difference in unplanned cesarean for TOLAC by provider type. The 19% relative decrease in unplanned cesarean that we observed for obstetrician management could become clinically important if the rate of unplanned cesarean or the number of TOLAC attempts were very high. We found no national data on the number of TOLAC deliveries managed by CNMs, but our findings suggest that increasing opportunities for midwives to manage low-risk TOLAC patients could be a safe and effective means to improve vaginal birth rates. A cultural and ideological shift towards offering TOLACs may be a notable obstacle, however, as fewer providers and hospitals are allowing TOLACs, some women may be unaware of the opportunity, and some reports suggest that fewer women overall are requesting VBAC.(6, 8–11, 33) Our data were collected prior to the publication of specific guidelines to reduce primary cesarean section.(34) However, those guidelines are not directly applicable to the TOLAC population, raising the possibility that new guidelines to reduce labor interventions specifically for TOLAC could improve vaginal delivery success. The use of Pitocin and regional anesthesia were significantly lower for CNM patients in our cohort, consistent with other studies(22) although a recent meta-analysis found no difference in Pitocin use by CNMs.(32)
This study has several strengths, including a large cohort from which we selected a low-risk sample with prospective data collection of both maternal and neonatal data. We evaluated an extensive set of maternal and pregnancy characteristics and used an established VBAC success calculator (i.e., Grobman score) for risk stratification. There was no loss to follow up as data were collected during admission for labor and birth. We recognize that intrapartum provider care could have switched from midwife to obstetrician during labor and for all unplanned cesareans, but we analyzed as intention-to-manage and the rate of patient transfer was modest. Providers were part of group practices within a single institution, reducing confounding due to variable individual, institutional, or time-sensitive factors. Our results were consistent after adjusting for maternal characteristics that were different by provider type, and a separate case-control sub-analysis gave similar findings. Our conclusions may be generalizable to healthy patients in similar collaborative centers with close working relationships between obstetricians and CNMs.
The study has some important limitations. First, it is retrospective, and there may be unrecognized assignment bias. We cannot discern women who may have self-selected provider type, though the demographics by provider were similar. We were unable to discriminate between provider effects due to prenatal care versus intrapartum management, and we have no long-term data on outcomes. It is possible that some women appeared more than once in our cohort, but due to the deidentified dataset we could not adjust for repeat enrollment. The high VBAC success rate could mean that healthy, low-risk patients were well-selected for TOLAC and therefore more successful. Alternately, the high VBAC success could be due to under-recording of unsuccessful TOLAC attempts (i.e. some cases could have been recorded in the medical record as repeat cesarean instead of attempted TOLAC because the delivery type would be cesarean or VBAC). However, matched case-control sub-analysis gave similar results by provider type, even after adjustment. The high VBAC success rate, therefore, may simply be due to an institutional culture that strongly encourages TOLAC but recommends against it for those patients with low chance of success. Furthermore, patient preference for an elective repeat cesarean could have removed poorer candidates from attempting TOLAC, leaving candidates with a higher likelihood of TOLAC success in our cohort. We recognize that our findings are only generalizable to other large well-integrated nurse midwifery practices with obstetric support, which may not be feasible in all community settings where immediate surgical back-up is not always available. TOLAC is offered widely by both obstetric and midwifery providers at our institution and, interestingly, unit-level presence of midwives has been associated with increased TOLAC attempts but not VBAC success.(35) Finally, we were unable to assess maternal motivation and TOLAC risk tolerance by provider group in our study, as midwifery patients might be more motivated for TOLAC. Prior studies have also found that, among acceptable candidates, managing providers not only influence patient decisions to undergo TOLAC but also TOLAC success.(33, 36)
Overall, there was no significant difference in unplanned cesarean rate by provider type during attempted TOLAC. Patients cared for by CNMs had fewer interventions in labor but similar VBAC success and morbidity. Although protocols are established to avoid the first primary cesarean,(34) the United States’ health care system’s capacity to accommodate TOLAC could be expanded by developing hospital guidelines to encourage more provider types to offer TOLAC and emphasize collaborative practice models. Furthermore, our experience suggests that other well-staffed institutions could potentially increase their TOLAC success with rigorous counseling and patient selection. With the understanding that TOLAC is a complex practice requiring increased resource availability, further investigation with high-quality trials of maternity care models to evaluate provider type and management approach is needed to decrease the rate of unplanned cesarean.
Supplementary Material
Financial Support:
National Institutes of Health (1F31NR014061, NSC), March of Dimes (NSC), SMFM-AAOGF Scholar Award (KJH).
Footnotes
Potential conflicts of interest/Disclosure of financial support: None of the authors have any personal, professional, or financial conflicts of interest to disclose.
Contributor Information
Matthew S. Fore, Penn State Health Milton S. Hershey Medical Center, Obstetrics and Gynecology, 500 University Drive, Hershey PA 17033,.
Amanda A. Allshouse, University of Utah School of Medicine, Obstetrics and Gynecology, Maternal Fetal Medicine, 30 N. 1900 E, Salt Lake City Utah 84132,.
Nicole S. Carlson, Emory University Nell Hodgson Woodruff School of Nursing, 1520 Clifton Road NE, Atlanta GA 30322,.
K. Joseph Hurt, University of Colorado School of Medicine, Obstetrics and Gynecology, Maternal Fetal Medicine & Reproductive Sciences, 12700 East 19th Avenue, MS 8613, Aurora CO 80045,.
REFERENCES
- 1.Hamilton BE, Martin JA, Osterman MJK, Rossen LM. Births: Provisional data for 2018. Natl Vital Stat Rap Rel 2019;007 2. Zhang J, Troendle J, Reddy U, et al. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol. 2011;203(4):326e1–326e10. [Google Scholar]
- 2.American College of Obstetricians and Gynecologists. Safe prevention of the primary cesarean delivery. Obstetric care consensus no. 1. Obstet Gynecol. 2014;123:693–711. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Troendle J, Reddy U, et al. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol. 2011;203(4):326e1–326e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uddin SFG, Simon AE. Rates and Success Rates of Trial of Labor After Cesarean Delivery in the United States, 1990–2009. Matern Child Health J 2012;17(7):1309–1314. [DOI] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services (US DHHS), Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS), Division of Vital Statistics, Natality public-use data 2016–2017. CDC WONDER Online Database 2018. Accessed at http://wonder.cdc.gov/natality-expanded-current.html. on Sep 2, 2019 [Google Scholar]
- 6.Wells CE. Vaginal Birth After Cesarean Delivery: Views from the Private Practitioner. Semin Perinatol. 2010;34(5):345–50. [DOI] [PubMed] [Google Scholar]
- 7.Coleman VH, Erickson K, Schulkin J, Zinberg S, Sachs BP. Vaginal birth after cesarean delivery: practice patterns of obstetrician-gynecologists. J Reprod Med. 2005;50(4):261–6. [PubMed] [Google Scholar]
- 8.Barger MK, Dunn JT, Bearman S, DeLain M, Gates E. A survey of access to trial of labor in California hospitals in 2012. BMC Pregnancy Childbirth. 2013;13(83). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sargent J, Caughey AB. Vaginal Birth After Cesarean Trends: Which Way Is the Pendulum Swinging? Obstet Gynecol Clin North Am 2017;44(4):655–66. [DOI] [PubMed] [Google Scholar]
- 10.Leeman LM, Beagle M, Espey E, Ogburn T, Skipper B. Diminishing Availability of Trial of Labor After Cesarean Delivery in New Mexico Hospitals. Obstet Gynecol 2013;122(2, PART 1):242–247. [DOI] [PubMed] [Google Scholar]
- 11.Grobman WA, Lai Y, Landon MB, Spong CY, Rouse DJ, Varner MW, et al. The change in the VBAC Rate: An epidemiologic analysis. Paediatr Perinat Epidemiol. 2011;25(1):37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landon MB and Grobman WA for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. What we have learned about trial of labor after cesarean delivery from the maternal-fetal medicine units cesarean registry. Semin Perinatol. 2016;40:281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curtin SC, Gregory KD, Korst LM, Uddin SFG. Maternal morbidity for vaginal and cesarean deliveries, according to previous cesarean history: New data from the birth certificate, 2013. Natl Vital Stat Rep 2015;64(4). [PubMed] [Google Scholar]
- 14.Lieberman E, Ernst EK, Rooks JP, Stapleton S, Flamm B. Results of the national study of vaginal birth after cesarean in birth centers. Obstet Gynecol 2004;104(5):933–42. [DOI] [PubMed] [Google Scholar]
- 15.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin no. 205: Vaginal Birth After Cesarean. Obstet Gynecol. 2019;133(2):e110–27. [DOI] [PubMed] [Google Scholar]
- 16.Guise JM, Denman MA, Emeis C, Marshall N, Walker M, Fu RW, et al. Vaginal Birth After Cesarean New Insights on Maternal and Neonatal Outcomes. Obstet Gynecol 2010. Jun;115(6):1267–78. [DOI] [PubMed] [Google Scholar]
- 17.Spong CY, Landon MB, Gilbert S, Rouse DJ, Leveno KJ, Varner MW, et al. Risk of uterine rupture and adverse perinatal outcome at term after cesarean delivery. Obstet Gynecol 2007;110(4):801–7. [DOI] [PubMed] [Google Scholar]
- 18.Black M, Bhattacharya S, Philip S, Norman JE, McLernon DJ. Planned Repeat Cesarean Section at Term and Adverse Childhood Health Outcomes: A Record-Linkage Study. Plos Med 2016;13(3):e1001973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhle S, Tong OS, Woolcott CG. Association between caesarean section and childhood obesity: a systematic review and meta-analysis. Obes Rev. 2015;16(4):295–303. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert SA, Grobman WA, Landon MB, Spong CY, Rouse DJ, Leveno KJ, et al. Cost-Effectiveness of Trial of Labor after Previous Cesarean in a Minimally Biased Cohort. Am J Perinatol 2013;30(1):11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlson NS, Corwin EJ, Hernandez TL, Holt E, Lowe NK, Hurt KJ. Association between provider type and cesarean birth in healthy nulliparous laboring women: A retrospective cohort study. Birth. 2018;45(2):159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johantgen M, Fountain L, Zangaro G, Newhouse R, Stanik-Hutt J, White K. Comparison of labor and delivery care provided by certified nurse-midwives and physicians: a systematic review, 1990 to 2008. Womens Health Issues. 2012;22(1):e73–81. [DOI] [PubMed] [Google Scholar]
- 23.Souter V, Nethery E, Kopa ML, Wurz H, Sitcov K, Caughey AB. Comparison of midwifery and obstetric care in low-risk hospital births. Obstet Gynecol 2019;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. The International Classification of adult underweight, overweight and obesity according to BMI. 2016. [cited 2016 08/25/2016]; Available from: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.
- 25.Institute of Medicine Guidelines, Rasmussen KM, Yaktine AL, eds. Weight gain during pregnancy: reexamining the guidelines. Washington (DC): National Academies Press (US) 2009. [PubMed] [Google Scholar]
- 26.Grobman WA, Lai Y, Landon MB, Spong CY, Leveno KJ, Rouse DJ, et al. Development of a nomogram for prediction of vaginal birth after cesarean delivery. Obstet Gynecol. 2007;109(4):806–12 [DOI] [PubMed] [Google Scholar]
- 27.Landon MB, Leindecker S, Spong CY, Hauth JC, Bloom S, Varner MW, et al. The MFMU Cesarean Registry: Factors affecting the success of trial of labor after previous cesarean delivery. Am J Obstet Gynecol 2005;193(3):1016–23. [DOI] [PubMed] [Google Scholar]
- 28.Srinivas SK, Stamilio DM, Stevens EJ, Odibo AO, Peipert JF, Macones GA. Predicting failure of a vaginal birth attempt after cesarean delivery. Obstet Gynecol. 2007;109(4):800–5. [DOI] [PubMed] [Google Scholar]
- 29.Edmonds JK, Yehezkel R, Liao X, Simas TAM. Racial and ethnic differences in primary, unscheduled cesarean deliveries among low-risk primiparous women at an academic medical center: a retrospective cohort study. BMC Pregnancy Childbirth 2013;13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altman MR, Murphy SM, Fitzgerald CE, Andersen HF, Daratha KB. The Cost of Nurse-Midwifery Care: Use of Interventions, Resources, and Associated Costs in the Hospital Setting. Womens Health Issues 2017;27(4):434–40. [DOI] [PubMed] [Google Scholar]
- 31.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: Final data for 2017. Natl Vital Stat Rep 2018;67(8). [PubMed] [Google Scholar]
- 32.Sandall J, Soltani H, Gates S, Shennan A, Devane D. Midwife-led continuity models versus other models of care for childbearing women (Review). Cochrane Database Syst Rev 2016(4. Art. No.: CD004667). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Metz TD, Stoddard GJ, Henry E, Jackson M, Holmgren C, Esplin S. How do good candidates for trial of labor after cesarean (TOLAC) who undergo elective repeat cesarean differ from those who choose TOLAC? Am J Obstet Gynecol 2013;208(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spong CY, Berghella V, Wenstrom KD, Mercer BM, Saade GR. Preventing the first cesarean delivery: summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists Workshop. Obstet Gynecol 2012;120(5):1181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlson NS, Neal JL, Tilden EL, et al. Influence of midwifery presence in United States centers on labor care and outcomes for low-risk parous women: A Consortium on Safe Labor Study. Birth 2018;00:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wingert A, Johnson C, Featherstone R, Sebastianski M, Hartling L, Wilson RD. Adjunct clinical interventions that influence vaginal birth after cesarean rates: systematic review. BMC Pregnancy Childbirth. 2018;18(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.