Abstract
Alexithymia is a personality trait characterized by difficulties identifying feelings (DIF), describing feelings (DDF), and externally oriented thinking (EOT). Alexithymia has been associated with poorer memory, at least for emotive materials, and recently, with executive and neural dysfunction. Aging is also accompanied by poorer memory and executive function (EF), neural dysfunction, and increasing alexithymia. Thus, the hypothesis of a general cognitive impairment in alexithymia, particularly in elders, needs investigation. Three large, independent, cross-sectional experiments (n = 296, 139 and 121, respectively) investigated memory and EF in healthy adults, ranging from young to old adulthood, with age, sex, and the three Toronto Alexithymia Scale-20 subscales (DIF, DDF, EOT) as predictors in hierarchical regressions. Across studies, alexithymia contributed to poorer memory (via EOT) and EF (via DIF), in younger and older adults. Additionally, these effects occurred in non-emotive contexts with neutral stimuli. Moreover, although memory was worse with greater age and poor EF contributed to poor memory, those who had both high EOT and poor EF had particularly poor memory. Thus, alexithymia (particularly via high DIF or high EOT) is a risk factor for age-related cognitive decline. Further research should clarify the direction and nature of these complex relationships.
Keywords: alexithymia, aging, episodic memory, executive functioning
Alexithymia is a personality trait associated with difficulties identifying feelings (DIF), difficulties describing feelings (DDF), and externally oriented thinking (EOT) (Bagby, Parker, & Taylor, 1994; Bagby, Taylor, & Parker, 1994; Nemiah, 1977; Sifneos, 1973; Taylor, 2000). People with high alexithymia scores (HA) have deficits in both the cognitive processing and regulation of emotions (Lane et al., 1996; Swart, Kortekaas, & Aleman, 2009), likely via poor emotional awareness, recognition, and differentiation (da Silva, Vasco, & Watson, 2017); and slower, less accurate emotion identification, suggesting poorer automatic attentional processing (Vermeulen, Luminet, & Corneille, 2006) versus people with low alexithymia scores (LA). Typically, attentional resources are directed toward salient content in the environment, such as negative or arousing stimuli, which enhances memory for the salient content (Mather & Sutherland, 2011; Nielson & Correro, 2017). However, HA cannot as readily process emotional information, exhibiting impairment in perception, attention allocation, and regulation related to emotion (Aftanas, Varlamov, Reva, & Pavlov, 2003; da Silva et al., 2017; Luminet, Vermeulen, Demaret, Taylor, & Bagby, 2006a; Swart et al., 2009; van der Velde et al., 2015; Vermeulen et al., 2006), and interpreting emotions at a behavioural or physiological level (Kano & Fukudo, 2013; Moriguchi & Komaki, 2013).
Evidence is accumulating that HA contributes to deficits in learning and memory specifically for emotional information and contexts (Dressaire et al., 2015; Jacob & Hautekeete, 1998; Luminet, Vermeulen, Demaret, Taylor, & Bagby, 2006b; Meltzer & Nielson, 2010; Suslow, Kersting, & Arolt, 2003; Vermeulen, Domachowska, & Nielson, 2018; Vermeulen & Luminet, 2009; Vermeulen, Toussaint, & Luminet, 2010), while memory for neutral memoranda appears intact. Yet, such studies typically examined neutral material in the context of emotion. At least one study of young adults (n=85) showed evidence of poorer memory in HA for neutral memoranda in a neutral context (Nielson & Meltzer, 2009). Story and complex figure recall have also been negatively correlated with alexithymia in a sample of 20 older adults (Onor, Trevisiol, Spano, Aguglia, & Paradiso, 2010), and poorer short- and long-term memory in HA was reported for pictures of people in social situations, despite comparable verbal ability across groups and samples of only 12 per group (DiStefano & Koven, 2012). These studies hint at a general memory impairment in alexithymia. Considering alexithymia facets, DIF has been implicated in impaired memory for emotional material (Luminet et al., 2006b; Vermeulen & Luminet, 2009), though inconsistently (Dressaire et al., 2015). Similarly, EOT has been related to poorer memory for emotive words (Dressaire et al., 2015; Meltzer & Nielson, 2010) but better memory for neutral words within an emotive context (Meltzer & Nielson, 2010; Vermeulen & Luminet, 2009). Yet, in neutral context, there is evidence EOT associates with impairment, particularly in elders (Onor et al., 2010). Given the paucity of studies, the typically small samples, and little study of specific alexithymia facets, further research is needed in neutral contexts to examine whether a more general memory deficit exists in alexithymia.
Executive functions are the higher-order cognitive processes involved in goal-directed behaviour, such as inhibition, fluency, set-shifting, and performance monitoring (Diamond, 2013). Importantly, executive functioning (EF) impacts the utilisation and effectiveness of other cognitive abilities, including learning and memory (Duff, Schoenberg, Scott, & Adams, 2005; Hill, Alosco, Bauer, & Tremont, 2012). A growing literature suggests executive dysfunction in HA, shown on measures ranging from fluency (Henry, Phillips, Crawford, Theodorou, & Summers, 2006; Santorelli & Ready, 2015; Wood & Williams, 2007) to problem solving (Onor et al., 2010); sequencing, set-shifting, and conflict processing (Wood & Williams, 2007); inhibition (Zhang et al., 2012; Zhang et al., 2011); and self-report (Koven & Thomas, 2010). Ultimately, executive dysfunction may contribute to emotion processing difficulties and related memory deficits in alexithymia. Moreover, most studies of EF employ non-emotive, neutral stimuli (except Zhang et al., 2012), hinting at a general cognitive deficit in alexithymia. However, this literature is small, using small samples and varied measures and approaches; some examined neurologically-induced alexithymia (e.g., Henry et al., 2006; Wood & Williams, 2007). Results are mixed, even across relatively comparable tasks (e.g., Henry et al., 2006; Lamberty & Holt, 1995; Santorelli & Ready, 2015). Finally, few studies have directly examined the facets of alexithymia as related to EF. The limited existing work suggests DIF and DDF are associated with executive dysfunction (Correro, Marra, Reiter, Byers, & Nielson, 2016; Henry et al., 2006; Koven & Thomas, 2010; Santorelli & Ready, 2015). More research examining EF with larger samples and examining the specific facets of EF are particularly needed.
Cognitive dysfunction in alexithymia is apparent across the lifespan (e.g., Correro et al., 2016; Lamberty & Holt, 1995; Onor et al., 2010; Santorelli & Ready, 2015). Yet, after age 30, increasing age is associated with increasing alexithymia (Mattila, Salminen, Nummi, & Joukamaa, 2006; Onor et al., 2010), as well as with neural dysfunction, particularly involving frontal lobe function and circuits, that results in declining EF and memory over time (Charlton et al., 2008; Goerlich & Aleman, 2018; Vermeulen et al., 2018). Thus, it is essential to delineate the unique contributions of alexithymia and age to cognitive functioning, and to better isolate the facets of alexithymia that result in cognitive deficits, to help clarify and address the underlying mechanisms of cognitive effects of alexithymia.
The primary objective of the current experiments was to evaluate the relationships between alexithymia, memory and EF in relatively large young and older adult samples, focusing on general cognitive functioning rather than using emotive contexts or memoranda. We used separate, non-overlapping samples for three experiments; each was a secondary analysis of data from prior protocols.1 Using non-emotive stimuli and measuring alexithymia with a standard and frequently used measure (the Toronto Alexithymia Scale—20 [TAS-20], Bagby, Parker, et al., 1994; Bagby, Taylor, et al., 1994), we first examined whether a laboratory word memory task would be predicted by alexithymia after accounting for age and sex in a large normative sample of young adults (Experiment 1). In Experiment 2 we examined whether executive functioning, using standardized neuropsychological tests, would be predicted by alexithymia in a large sample including young and older adults. Finally, in Experiment 3 we examined whether alexithymia would predict memory and EF, using standardized neuropsychological tests in young and older adults, and the contribution of alexithymia to the memory-EF relationship.
Experiment 1
Memory is altered by alexithymia in emotional contexts (see Vermeulen et al., 2018). However, studies with larger samples are needed to verify whether memory alterations in alexithymia might be apparent for neutral memoranda, particularly in neutral contexts, and to further probe specific facet(s) of alexithymia. In Experiment 1, young adults completed a verbal, non-emotive memory task that used nouns as memoranda, with long-term retention assessed 60 minutes later. Based on very limited prior research we hypothesized that, after accounting for age and sex, memory for neutral words in a neutral context would be impaired by alexithymia (DiStefano & Koven, 2012; Nielson & Meltzer, 2009; Onor et al., 2010), speculating that EOT might be responsible (Onor et al., 2010) due to its more cognitive role in alexithymia (Vermeulen & Luminet, 2009).
Method
Participants
Young adults (n=297) were recruited from university classes; those ≥18 years and with English as first language (EFL) were included. One subject had incomplete TAS-20 data, leaving a final sample of 296 (214 female). Descriptive statistics are shown in Table 1.
Table 1.
Experiment 1 (n=296, 214 female), Descriptive Statistics.
| Mean | SD | |
|---|---|---|
| Age (years) | 19.68 | 1.93 |
| BSI-Depression | 0.84 | 0.64 |
| BSI-Anxiety | 0.91 | 0.62 |
| TAS-20 Total | 44.85 | 11.06 |
| Difficulty Identifying Feelings | 13.50 | 5.27 |
| Difficulty Describing Feelings | 12.63 | 4.80 |
| Externally Oriented Thinking | 18.80 | 4.78 |
| Immediate Recall (of 30) | 13.58 | 3.80 |
| Delayed Recognition Hits (%) | 67.90 | 15.80 |
| Delayed Recognition False Alarms (%) | 5.50 | 6.10 |
| Delayed Recognition Sensitivity (d’) | 2.58 | 1.33 |
| Delayed Recognition Response Bias (C) | 0.71 | 0.36 |
Notes: BSI = Brief Symptom Inventory; TAS-20 Total = Toronto Alexithymia Scale-20.
Materials
The memory task used 30 high-imagery nouns (>6.0 on a scale of 1–7, Paivio, Yuille, & Madigan, 1968; e.g., “butterfly,” “queen,” “house”) presented at three-second intervals (white on blue) with instructions to remember the words. Free recall was tested immediately to ensure encoding. Delayed retention was tested by recognition using 140 quasi-randomly ordered prompts; 30 targets, 110 distracters (Nielson, Yee, & Erickson, 2005). A 60-min delay targeted long-term memory and helped prevent recognition ceiling effects. Table 1 provides raw recognition metrics (hits, false alarms), sensitivity (i.e., degree of overlap between signal and noise distributions, d’= z(false alarms)–z(hits)), and response bias, C= −(z(hits)+z(false alarms))/2; negative=liberal (yes), positive=conservative (no) (Stanislaw & Todorov, 1999).
Alexithymia was measured using the 20-item Toronto Alexithymia Scale (TAS-20; Bagby, Parker, et al., 1994; Bagby, Taylor, et al., 1994), using a five-point scale (possible scores=20–100). Three non-orthogonal subscales include Difficulty Identifying Feelings (DIF; range=7–35), Difficulty Describing Feelings (DDF; range=5–25), and Externally Oriented Thinking (EOT; range=8–40). TAS-20 has good reliability, convergent/concurrent and criterion validity (e.g., Parker, Taylor, & Bagby, 2003; Taylor, Bagby, & Luminet, 2000; Taylor, Bagby, & Parker, 2003). Internal consistency (IC) is typically ≥0.70 with EOT lowest (Parker et al., 2003); current study IC: Total=0.834, DIF=0.827, DDF=0.821, EOT=0.668. Due to relevance to alexithymia, the depression and anxiety subscales of the Brief Symptom Inventory (BSI) are shown (Table 1). The 53-item BSI measures nine symptom categories (depression and anxiety raw scores = average of 6 items, on scale of 0 (none) to 4 (severe)); it has acceptable reliability, convergent/concurrent and criterion validity, and IC>0.70 (Derogatis & Melisaratos, 1983)2.
Design and Procedure
Participants were tested individually, completing a demographic survey followed by the memory task. After encoding and immediate free recall, a delay of 60 minutes was filled with multiple surveys, including the TAS-20, followed by recognition testing1. Correlations were examined with Pearson r, or Spearman’s rho where non-normality existed. Hierarchical regressions were performed predicting delayed memory measures using age (years) and sex in Step 1, and all three TAS-20 subscales in Step 2 as predictors (SPSS v.24). Given the correlations amongst the variables of interest and the non-orthogonal TAS-20 subscales, multicollinearity (tolerance, variance inflation factor) prevented adding all interaction terms to these models; only targeted interactions were explored. A priori power analysis for hierarchical regression (2+3 predictors; medium effect (.15), power≥0.80, p<0.05) indicated a sample of 78; adding two predictors (i.e., interactions) suggested a sample of 90 (Soper, 2019).
Results and Discussion
Bivariate correlations are shown in Table 2. Outcomes (memory) did not correlate significantly with age, depression or anxiety in this sample. Regarding alexithymia, only EOT correlated significantly with memory (hits and d’), while depression and anxiety correlated with DIF and DDF. The TAS-20 subscores in this large but age-restricted sample were log-transformed prior to regression to improve normality.
Table 2.
Experiment 1, Bivariate Correlations.
| Age | Sex | BSID | BSIA | DIF | DDF | EOT | IR | Hits | FA | d’ | C | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | - | |||||||||||
| Sex | 0.00 | - | ||||||||||
| BSID | −0.15 | 0.15 | - | |||||||||
| BSIA | −0.09 | 0.15 | 0.61 | - | ||||||||
| TAS DIF | −0.18 | 0.00 | 0.51 | 0.50 | - | |||||||
| TAS DDF | −0.22 | 0.00 | 0.37 | 0.32 | 0.52 | - | ||||||
| TAS EOT | −0.17 | −0.10 | 0.01 | −0.03 | 0.12 | 0.37 | - | |||||
| IR | −0.08 | −0.14 | 0.06 | 0.04 | 0.06 | −0.01 | −0.13 | -- | ||||
| Hits | 0.03 | 0.24 | 0.09 | 0.06 | 0.04 | 0.03 | −0.14 | 0.54 | - | |||
| FA | 0.05 | −0.11 | 0.01 | 0.03 | 0.06 | 0.01 | 0.08 | −0.37 | −0.22 | - | ||
| d’ | −0.05 | 0.23 | 0.06 | 0.02 | 0.01 | 0.01 | −0.15 | 0.55 | 0.82 | −0.74 | - | |
| C | −0.01 | −0.11 | −0.09 | −0.07 | −0.08 | −0.03 | 0.0 | 0.07 | −0.70 | −0.55 | −0.16 | - |
Notes: < .05; <.01; Pearson r except with sex (Spearman’s rho); TAS = Toronto Alexithymia Scale-20; DIF= Difficulty Identifying Feelings; DDF=Difficulty Describing Feelings; EOT=Externally Oriented Thinking; BSI=Brief Symptom Inventory (D= Depression, A=Anxiety); IR: Immediate recall; FA=False Alarms; d’=sensitivity; C=response bias.
Hierarchical regression (see Table 3) produced a significant model predicting memory (d’) with sex significant in Step 1; sex, DIF and EOT were significant in Step 2. Similar but weaker models resulted for uncorrected hits and immediate recall. None of the study variables predicted false alarms (p>.13) or C (p>.17). Thus, men and those with higher DIF and EOT had poorer delayed memory, via sensitivity, for neutral words. Although depression and anxiety did not correlate with memory, including either added no significant prediction in any model (ps>.54), nor did they supplant TAS-20 predictors. Although immediate recall was significantly correlated with d’, including it in the model did not alter the relationship of TAS-20 to d’. Last, the interaction of sex and EOT as Step 3 was not significant (p=.54); other terms were unchanged by this addition. Thus, although females had better memory performance than males, EOT was associated with poorer memory for neutral words in both men and women.
Table 3.
Experiment 1, Hierarchical Regression Predicting Delayed Recognition Memory Sensitivity.
| Model Summary of Each Step | Contribution of Each Variable in Last Step | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | DR2 | F | p | B | SE | b | t | p | ||
| Hits | ||||||||||
| Step 1 | 0.051 | - | 7.82 | <0.01 | ||||||
| Age | −0.011 | 0.015 | −0.041 | −0.70 | 0.48 | |||||
| Sex | 0.236 | 0.065 | 0.209 | 3.65 | <0.01 | |||||
| Step 2 | 0.073 | 0.021 | 2.22 | 0.09 | ||||||
| DIF | 0.017 | 0.090 | 0.012 | 0.18 | 0.86 | |||||
| DDF | 0.079 | 0.088 | 0.065 | 0.90 | 0.37 | |||||
| EOT | −0.305 | 0.121 | −0.156 | −2.52 | 0.01 | |||||
| False Alarms | ||||||||||
| Step 1 | 0.014 | - | 2.05 | 0.13 | ||||||
| Age | 0.011 | 0.013 | 0.052 | 0.86 | 0.39 | |||||
| Sex | −0.099 | 0.056 | −0.102 | −1.75 | 0.08 | |||||
| Step 2 | 0.027 | 0.013 | 1.67 | 0.16 | ||||||
| DIF | 0.100 | 0.079 | 0.087 | 1.27 | 0.21 | |||||
| DDF | −0.069 | 0.077 | −0.066 | −0.90 | 0.37 | |||||
| EOT | 0.172 | 0.106 | 0.103 | 1.63 | 0.11 | |||||
| d′ (sensitivity) | ||||||||||
| Step 1 | 0.022 | - | 3.24 | 0.04 | ||||||
| Age | 0.091 | 0.911 | 0.006 | 0.10 | 0.92 | |||||
| Sex | 0.434 | 0.171 | 0.147 | 2.54 | 0.01 | |||||
| Step 2 | 0.064 | 0.042 | 3.92 | <0.01 | ||||||
| DIF | −0.512 | 0.236 | −0.146 | −2.17 | 0.03 | |||||
| DDF | 0.434 | 0.231 | 0.135 | 1.88 | 0.06 | |||||
| EOT | −0.969 | 0.315 | −0.191 | −3.07 | <0.01 | |||||
| C (response bias) | ||||||||||
| Step 1 | 0.012 | - | 1.75 | 0.18 | ||||||
| Age | 0.000 | 0.009 | −0.002 | −0.04 | 0.97 | |||||
| Sex | −0.068 | 0.039 | −0.104 | −1.77 | 0.08 | |||||
| Step 2 | 0.020 | 0.008 | 1.18 | 0.32 | ||||||
| DIF | −0.058 | 0.054 | −0.074 | −1.08 | 0.28 | |||||
| DDF | −0.005 | 0.053 | −0.007 | −0.10 | 0.92 | |||||
| EOT | 0.066 | 0.072 | 0.059 | 0.92 | 0.36 | |||||
Notes: < .05; <.01; Toronto Alexithymia Scale-20: DIF = Difficulty Identifying Feelings, DDF = Difficulty Describing Feelings, EOT = Externally Oriented Thinking.
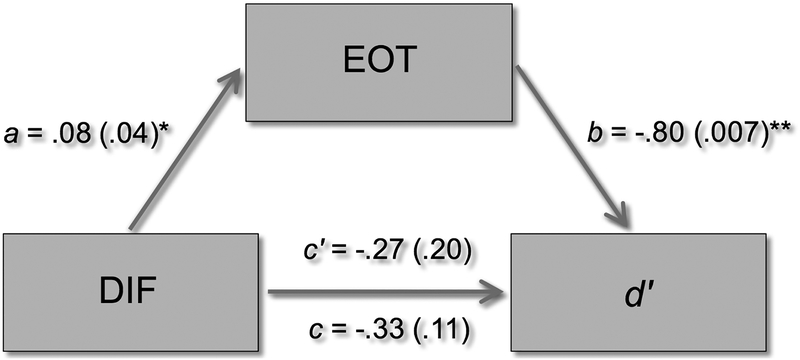
The contribution of both EOT and DIF to the model predicting d’, despite the lack of correlation of DIF with d’, warranted interrogation with bootstrapped mediation analysis (PROCESS 3.0; Hayes, 2018). Figure 1 demonstrates that EOT mediated the relationship of DIF to memory. Consistent with the lack of bivariate correlation, the direct effect of DIF on d’ was not significant (B=−.33, SE=.20, p=.11). Thus, DIF did not contribute to predicting d’ independently from EOT. Instead, the shared variance of DIF with EOT appeared to cause the significance of DIF in the model. Thus, the relationship of alexithymia to memory was primarily contributed by EOT.
Figure 1.
In Experiment 1, both Difficulty Identifying Feelings (DIF) and Externally Oriented Thinking (EOT) were significant predictors of recognition memory (d′) after accounting for age and sex. In this post-hoc mediation model (showing coefficient (p) for each path), the total effect of DIF on d′ (path c) is not significant (consistent with the lack of bivariate correlation between them; see Table 2). Instead, there is a significant relationship between DIF and EOT (path a) and between EOT and d′ (path b), which results in a weaker direct effect than total effect of DIF on d′ (i.e., path c′). This demonstrates mediation. That is, the effect of DIF on d′ did not occur independently from the effect of EOT on d′. Instead, EOT mediated the relationship between DIF and d′; EOT was the primary contributor to reduced memory in alexithymia.
Experiment 1 sought to examine the contribution of alexithymia to long-term memory in young adults for non-emotive memoranda. Through EOT, alexithymia contributed to reduced ability to retrieve neutral words after one hour. The proportion of variance captured by age and alexithymia is small but significant in this young sample. Alexithymia specifically contributed to sensitivity not attributable to depression, anxiety, or encoding. This is a novel finding. Previous studies have shown alexithymia results in poorer memory for emotive memoranda, attributing the effects to stimulus relevance (Luminet et al., 2006b; Meltzer & Nielson, 2010) and emotional context (Nielson & Meltzer, 2009; Vermeulen & Luminet, 2009; Vermeulen et al., 2010). Our results, using a larger than typical sample, suggest that EOT contributes to general difficulty distinguishing targets (“signal”) from foils (“noise”); contextual and personal salience effects in alexithymia may be additive rather than exclusive in memory processing and retrieval.
Experiment 2
Executive functioning declines (e.g., Charlton et al., 2008) while alexithymia increases during older age (Mattila et al., 2006), making aging an important context for evaluating cognitive relationships with alexithymia. A deficit in EF, with specific tasks varying by study, has been shown in a handful of alexithymia studies of neurologically altered and normative samples of younger and older adults (Henry et al., 2006; Koven & Thomas, 2010; Santorelli & Ready, 2015; Zhang et al., 2012; Zhang et al., 2011). Experiment 2 therefore sought to examine alexithymia and EF in a large, normative, cognitively intact sample including young to old adults. We hypothesized that a general executive factor, computed from multiple standard tasks of EF, would be predicted by both age and alexithymia. Based on Experiment 1, this small literature, and a small preliminary study (Correro et al., 2016), we hypothesized that DIF and DDF would specifically predict EF.
Method
Participants
Participants (144 (97 female)) were healthy, EFL, and cognitively intact adults: 40 young adults (18–35 years), and 104 older adults (48–86 years; these drawn from two different samples, targeting healthy adults ≥45 years, although no one 45–47 years volunteered). Participants reported no history of medical, neurological, psychiatric, or substance abuse conditions; hypertension was controlled. Older adults were screened for intact cognition (Mini-Mental State Exam (MMSE), Folstein, Folstein, & McHugh, 1975), ≥26/30). Five participants were missing EF score(s) due to data or record errors, leaving a final sample of 139. Descriptive statistics are in Table 4.
Table 4.
Experiment 2, Descriptive Statistics.
| All (n=139) | Young (n=38) | Older (n=101) | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | p | |
| Age (yrs) | 57.53 | 24.95 | 19.89 | 2.79 | 71.69 | 10.75 | <0.001 |
| Age range (yrs) | 18–35 | 48–89 | |||||
| Sex (M/F) | 45/94 | 11/27 | 34/67 | 0.60 | |||
| Education (yrs) | 14.91 | 2.29 | 13.74 | 1.08 | 15.34 | 2.47 | <0.001 |
| BSI-Depression | 0.40 | 0.47 | 0.50 | 0.54 | 0.36 | 0.44 | 0.10 |
| BSI-Anxiety | 0.48 | 0.46 | 0.69 | 0.63 | 0.40 | 0.36 | 0.01 |
| Mini-Mental State Exam | -- | -- | -- | -- | 29.06 | 1.14 | -- |
| TAS-20 Total | 43.26 | 9.76 | 43.66 | 9.42 | 43.11 | 9.93 | 0.77 |
| Difficulty Identifying Feelings | 12.64 | 4.39 | 13.26 | 4.93 | 12.41 | 4.18 | 0.31 |
| Difficulty Describing Feelings | 11.47 | 3.71 | 12.55 | 3.97 | 11.07 | 3.52 | 0.05 |
| Externally Oriented Thinking | 19.14 | 4.42 | 17.84 | 3.98 | 19.63 | 4.49 | 0.03 |
| Trail-making A (sec) | 28.31 | 10.29 | 20.29 | 5.01 | 31.31 | 10.18 | <0.001 |
| Trail-making B (sec) | 68.89 | 26.15 | 59.55 | 19.95 | 72.41 | 27.41 | 0.003 |
| Symbol-digit Modalities | 52.08 | 11.88 | 64.16 | 9.21 | 47.53 | 9.32 | <0.001 |
| Category Fluency | 20.77 | 4.65 | 22.66 | 3.95 | 20.06 | 4.71 | 0.002 |
Notes: < .05; <.01; TAS-20 Total = Toronto Alexithymia Scale-20 total score; BSI = Brief Symptom Inventory; p values correspond to between-group t-tests except sex (X2).
Materials
Alexithymia was assessed using the TAS-20 (IC: Total=0.838, DIF=0.839, DDF=0.726, EOT=0.652). Depression and anxiety were measured using BSI subscales, as in Experiment 1 (Derogatis & Melisaratos, 1983)2. EF was measured using standardized tests commonly included in neuropsychological assessment: the Trail-making Tests (TMT, A and B; Reitan, 1955), written Symbol-Digit Modalities Test (SDMT; Smith, 1991), and category fluency (animals; CF). TMT requires connecting numbered circles in order (TMT-A) and connecting lettered and numbered circles in alternation (TMT-B) as quickly as possible; it measures visual search, attention, processing speed, sequencing, and switching/mental flexibility. SDMT requires providing digits corresponding to symbols, as indicated by a key, as quickly as possible; it measures visual search, attention, processing speed, and switching/mental flexibility. Category fluency requires providing as many unique exemplars of a category as possible in one minute; it measures semantic access and search, speed, retrieval strategies, and flexibility. Each of these further requires a degree of inhibitory control (see Lezak, Howieson, Loring, & Fischer, 2004). Principal components analysis (PCA) was used to extract a single EF factor, capturing the EF commonalities across the various tests (eigenvalue=2.4); it accounted for 60% of variance (loadings .62 (CF) to .88 (TMT-A); positive scores reflect poorer performance).
Design and Procedure
Participants were tested individually, completing a demographic survey, EF tests and surveys, including the TAS-201. Hierarchical regression was performed as in Experiment 1, but with EF as the dependent variable (power analysis: n needed = 78; see Experiment 1).
Results and Discussion
Bivariate correlations are shown in Table 5. All TAS-20 subscales were inter-correlated. Age correlated only with EOT. Notably, no alexithymia facets correlated with age within young subjects, but all facets correlated with age within older adults (DIF=0.22, DDF 0.38, EOT=0.22, all ps<.025). Depression and anxiety correlated with TAS-20 DIF and DDF, but not with EF. EF tests correlated significantly with age, DIF (SDMT excepted), and EOT (TMT-A, overall only), but only TMT-B correlated with DDF.
Table 5.
Experiment 2, Bivariate Correlations.
| Age | Sex | BSI-D | BSI-A | DIF | DDF | EOT | EF | |
|---|---|---|---|---|---|---|---|---|
| Age | - | |||||||
| Sex | 0.05 | - | ||||||
| BSI-D | −0.12 | 0.05 | - | |||||
| BSI-A | −0.25 | 0.01 | 0.63 | - | ||||
| DIF | −0.02 | 0.05 | 0.34 | 0.35 | - | |||
| DDF | −0.04 | 0.13 | 0.18 | 0.27 | 0.60 | - | ||
| EOT | 0.24 | 0.28 | −0.06 | 0.05 | 0.23 | 0.41 | - | - |
| TMT-A | 0.58 | 0.16 | −0.01 | −0.03 | 0.18 | 0.11 | 0.31 | 0.86 |
| TMT-B | 0.36 | 0.07 | −0.01 | −0.03 | 0.22 | 0.19 | 0.13 | 0.80 |
| SDMT | −0.66 | −0.22 | 0.05 | 0.10 | 0.03 | 0.04 | −0.15 | −0.78 |
| CF | −0.31 | −0.01 | −0.01 | 0.02 | −0.18 | −0.07 | −0.11 | −0.63 |
| EF | 0.64 | 0.15 | 0.01 | −0.04 | 0.17 | 0.10 | 0.24 | - |
Notes: < .05; <.01; Pearson r except with sex (Spearman’s rho); BSI=Brief Symptom Inventory (D=depression; A=anxiety); DIF= Difficulty Identifying Feelings; DDF=Difficulty Describing Feelings; EOT=Externally Oriented Thinking; TMT=Trail-making Tests; SDMT=Symbol-digit Modalities Test; CF=Category fluency (animals); EF= executive functioning factor score.
Hierarchical regression (see Table 6) resulted in a significant model predicting executive functioning with age (years) and sex in Step 1. Age, sex and DIF were each significant predictors in Step 2. Age-X-DIF and sex-X-DIF interactions were not significant (R2=.29, p<.001; age p<.01, sex p=.06, DIF p=.04, interaction ps .21−.79). Anxiety and depression did not add prediction or reduce DIF effects when included in the model (ps>.22). Thus, poorer executive functioning was apparent in older age, male sex, and in those with higher DIF, with none of these contributions dependent on the value of the others.
Table 6.
Experiment 2, Hierarchical Regression Predicting Executive Functioning
| Model Summary of Each Step | Contribution of Each Variable in Last Step | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | DR2 | F | p | B | SE | b | t | p | ||
| EF Factor | ||||||||||
| Step 1 | 0.39 | - | 43.15 | <.001 | ||||||
| Age | 0.03 | 0.00 | 0.60 | 8.64 | <.001 | |||||
| Sex | 0.29 | 0.15 | 0.14 | 2.01 | 0.05 | |||||
| Step 2 | 0.43 | 0.04 | 3.29 | 0.02 | ||||||
| DIF | 0.04 | 0.02 | 0.19 | 2.22 | 0.03 | |||||
| DDF | 0.00 | 0.03 | 0.01 | 0.12 | 0.91 | |||||
| EOT | 0.01 | 0.02 | 0.03 | 0.37 | 0.71 | |||||
Notes: < .05; <.01; Toronto Alexithymia Scale-20: DIF = Difficulty Identifying Feelings, DDF= Difficulty Describing Feelings, EOT = Externally Oriented Thinking; EF=Executive Functioning.
Experiment 2 is consistent with the few prior studies indicating reduced executive functioning ability in those with higher alexithymia. Specifically, prior studies suggest that HA have poorer ability to attend to, manipulate, and quickly respond to stimuli and abstract information (Correro et al., 2016; Santorelli & Ready, 2015; Zhang et al., 2011). For example, in one study, alexithymia did not impact basic attentional processes (e.g., alerting, orienting) during a flanker-type task, yet HA were significantly slower and less accurate in deciding whether one arrow in an array of other arrows was pointing in the same or opposite direction (Zhang et al., 2011). This, as with our findings, occurred with neutral stimuli, suggesting alexithymia is associated with a generalized EF deficit. Earlier study of participants with traumatic brain injuries also found a unique association between DIF and executive function deficits as indexed by fluency tasks (Henry et al., 2006). The current large, normative sample spanning the adult age spectrum showed that greater DIF (but not DDF) was specifically associated with poorer general EF; fluency was included in this factor. Both age and alexithymia (DIF) were independently related to EF. A previous study reported that worse verbal EF predicted alexithymia in a sample of young and older adults, but the effect was exclusive to older adults (Santorelli & Ready, 2015). Follow-up analysis of our data supported the previous study: DIF contributed to executive dysfunction at all adult ages, but its effects were greater in older age. Thus, DIF and poor EF are particularly important to consider as risk factors for age-related cognitive decline.
Experiment 3
Our first two experiments indicated that EF and memory are each impacted by aging and alexithymia (i.e., DIF and EOT, respectively). Experiment 3 sought to build upon these experiments. Regarding memory, delayed narrative recall was assessed instead of word recognition. Stories assess ‘everyday memory’, affording greater ecological validity than word lists and retrieval with less cuing than recognition (e.g., Wang, Daselaar, & Cabeza, 2017). Yet, narratives provide more contextual support than word lists, lessening the EF load during retrieval (Rubin, 2006), which could be assistive to older adults with higher alexithymia. The task also allowed standardized neuropsychological testing to be used for both memory and EF assessment. We hypothesized that Experiment 3 would replicate Experiments 1 and 2. We further explored the relationship of EF to memory performance and the role of alexithymia in that relationship.
Method
Participants
Participants (121 (81 female)) were healthy, EFL, and cognitively intact adults (34 young, 18–22 years; 87 older, 50–92 years, targeting healthy adults ≥45 years) drawn from two different samples within the university’s metropolitan area. Participants were screened as in Experiment 2. All participants completed the EF tests, while only one of the older samples (n=42, 50–87 years) completed memory testing. Descriptive statistics are in Table 7.
Table 7.
Experiment 3, Descriptive Statistics.
| All (n=121) | Young (n=34) | Older (n=87) | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | p | |
| Age (yrs) | 53.25 | 22.9 | 19.24 | 0.99 | 66.55 | 9.80 | 0.10 |
| Age range (yrs) | 18–92 | 18–22 | 50–92 | ||||
| Sex (M/F) | 40/81 | 15/19 | 25/62 | 0.11 | |||
| Education (yrs) | 15.03 | 2.77 | 12.72 | 1.01 | 15.93 | 2.71 | 0.01 |
| Mini-Mental State Exam | -- | -- | -- | -- | 28.93 | 1.37 | -- |
| Beck Depression Inventory | 4.03 | 4.21 | 5.65 | 5.40 | 3.40 | 3.48 | 0.03 |
| Beck Anxiety Inventory | 4.23 | 5.02 | 7.94 | 7.31 | 2.83 | 2.78 | <0.01 |
| TAS-20 Total Score | 41.37 | 10.91 | 45.18 | 11.26 | 39.78 | 10.42 | 0.04 |
| Difficulty Identifying Feelings | 11.79 | 5.02 | 13.32 | 5.13 | 11.19 | 4.87 | <0.01 |
| Difficulty Describing Feelings | 11.00 | 3.68 | 12.56 | 3.74 | 10.38 | 3.44 | 0.31 |
| Externally Oriented Thinking | 18.58 | 4.81 | 19.29 | 5.36 | 18.30 | 4.57 | 0.33 |
| Trail-making A (sec) | 30.14 | 12.04 | 28.41 | 11.63 | 30.82 | 12.19 | <0.01 |
| Trail-making B (sec) | 70.41 | 29.12 | 58.58 | 17.66 | 75.02 | 31.41 | 0.03 |
| Executive Factor | 0.00 | 1.00 | −0.31 | 0.80 | 0.12 | 1.05 | <0.01 |
| RBMT IR (raw) | 9.58 | 3.52 | 10.97 | 3.02 | 8.63 | 3.59 | <0.01 |
| RMBT DR (raw) | 8.38 | 3.2 | 9.52 | 2.96 | 7.46 | 3.12 | <0.01 |
Notes: < .05; <.01; TAS-20 = Toronto Alexithymia Scale: DIF =Difficulty Identifying Feelings, DDF = Difficulty Describing Feelings, EOT = Externally Oriented Thinking; Trails = Trail-making Tests; RBMT = Rivermead Behavioural Memory Test, IR = immediate recall; DR = 30-min delayed recall; p values correspond to between-group t-tests except sex (X2); *RBMT n=42.
Materials
Alexithymia was assessed using the TAS-20 (IC: Total=0.820, DIF=0.833, DDF=0.655, and EOT=0.716). Depression and anxiety were measured using the Beck Depression and Anxiety Inventories (BDI, BAI; raw score sum of 21 items, scale of 0 (none) to 3 (severe)), which have good reliability, convergent/concurrent and criterion validity, and IC (Beck & Steer, 1990; Beck, Steer, & Brown, 1996)3.
EF was measured using the TMT-A/B (Reitan, 1955); PCA extracted commonalities into a single EF factor (77% of variance; loadings=.88; positive standard score=poorer EF). Memory was assessed using the Rivermead Behavioural Memory Test (RBMT) story subtest, a standardized test of everyday memory with strong reliability and construct, clinical and ecological validity (Wilson, Cockburn, Baddeley, & Hiorns, 1989). Immediate recall assesses attention/encoding, while 30-minute delayed recall (standardized delay) examined long-term memory.
Design and Procedure
Participants were tested individually, completing a demographic survey, followed by the RBMT and TMT. Surveys, including the TAS-20 were administered during the delay1. Hierarchical regression was used, with the EF component score or memory score as the dependent variable (power analysis: n needed = 78, see Experiment 1). The Johnson-Neyman procedure was used (CAHOST, Carden, Holtzman, & Strube, 2017) to examine interaction effects.
Results and Discussion
Bivariate correlations among the study variables are shown in Table 8. EF significantly correlated with age, sex, and DIF. Memory significantly correlated with age, sex, and EF. Although age did not correlate with alexithymia total score across the full sample or in young alone (r=.17, p=.33), age and alexithymia significantly correlated in older adults alone (r=.25, p<.02).
Table 8.
Experiment 3, Bivariate Correlations.
| Age | Sex | BDI | BAI | DIF | DDF | EOT | Exec | IR | DR | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | -- | |||||||||
| Sex | .001 | -- | ||||||||
| BDI | −0.16 | −0.02 | -- | |||||||
| BAI | −0.41 | 0.13 | 0.36 | -- | ||||||
| DIF | −0.08 | −0.13 | 0.44 | 0.45 | -- | |||||
| DDF | −0.20 | −0.24 | 0.37 | 0.22 | 0.64 | -- | ||||
| EOT | −0.06 | −0.37 | 0.22 | 0.10 | 0.36 | 0.47 | -- | |||
| Exec | 0.37 | −0.22 | 0.10 | −0.07 | 0.24 | 0.14 | 0.10 | -- | ||
| IR | −0.43 | 0.23 | 0.20 | 0.20 | 0.15 | 0.18 | −0.13 | −0.40 | -- | |
| DR | −0.43 | 0.25 | 0.17 | 0.21 | 0.09 | 0.09 | −0.19 | −0.38 | 0.84 | -- |
Notes: < .05; <.01; Pearson r except sex (rho); N=121 (except IR, DR: N=76); BDI=Beck Depression Inventory; BAI=Beck Anxiety Inventory; Toronto Alexithymia Scale-20: DIF=Difficulty Identifying Feelings, DDF=Difficulty Describing Feelings, EOT = Externally Oriented Thinking; Exec = factor score of Trail-making Tests A and B; IR=immediate, DR=delayed recall on the Rivermead Behavioural Memory Tests (story subtest).
Hierarchical regression produced a significant model in which age and sex predicted EF in Step 1, with DIF adding significant variance in Step 2 (see Table 9). A post-hoc hierarchical regression analysis added the DIF interaction terms with age and sex in subsequent steps. Neither interaction term was significant (ps>.12), while age, sex and DIF remained significant as independent predictors. Anxiety and depression did not correlate with EF; adding either to the model had no effect (ps>.35). Thus, older age, male sex, and greater DIF each contributed to poorer executive functioning.
Table 9.
Experiment 3, Hierarchical Regression Predicting Executive Functioning.
| Model Summary of Each Step | Contribution of Each Variable in Last Step | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | DR2 | F | p | B | SE | b | t | p | |||
| Executive Function | |||||||||||
| Step 1 | 0.20 | - | 14.99 | <.001 | |||||||
| Age | 0.02 | 0.00 | 0.41 | 5.02 | <.001 | ||||||
| Sex | −0.57 | 0.18 | −0.27 | −3.15 | <.01 | ||||||
| Step 2 | 0.27 | 0.07 | 3.60 | 0.02 | |||||||
| DIF | 0.05 | 0.02 | 0.25 | 2.39 | 0.02 | ||||||
| DDF | 0.01 | 0.03 | 0.05 | 0.45 | 0.65 | ||||||
| EOT | −0.02 | 0.02 | −0.09 | −0.92 | 0.36 | ||||||
| Immediate Recall | |||||||||||
| Step 1 | 0.26 | - | 12.54 | <.001 | |||||||
| Age | −0.02 | 0.01 | −0.43 | −4.17 | <.001 | ||||||
| Sex | −0.47 | 0.23 | −0.22 | −2.04 | 0.05 | ||||||
| Step 2 | 0.31 | 0.05 | 1.81 | 0.15 | |||||||
| DIF | 0.02 | 0.03 | 0.08 | 0.56 | 0.58 | ||||||
| DDF | 0.05 | 0.04 | 0.18 | 1.25 | 0.22 | ||||||
| EOT | −0.05 | 0.03 | −0.22 | −1.87 | 0.07 | ||||||
| Delayed Memory (all) | |||||||||||
| Step 1 | 0.28 | - | 13.86 | <.001 | |||||||
| Age | −0.02 | 0.00 | −0.46 | −4.52 | <.001 | ||||||
| Sex | 0.45 | 0.23 | 0.21 | 1.97 | 0.05 | ||||||
| Step 2 | 0.33 | 0.05 | 1.82 | 0.15 | |||||||
| DIF | 0.02 | 0.03 | 0.09 | 0.69 | 0.50 | ||||||
| DDF | 0.04 | 0.04 | 0.13 | 0.93 | 0.36 | ||||||
| EOT | −0.05 | 0.03 | −0.25 | −2.12 | 0.04 | ||||||
| Delayed Memory (Older only) | |||||||||||
| Step 1 | 0.36 | - | 10.72 | <.001 | |||||||
| Age | −0.05 | 0.01 | −0.52 | −3.75 | <.001 | ||||||
| Sex | 0.33 | 0.34 | 0.15 | 0.96 | 0.34 | ||||||
| Step 2 | 0.50 | 0.14 | 3.34 | 0.03 | |||||||
| DIF | 0.01 | 0.04 | 0.06 | 0.34 | 0.74 | ||||||
| DDF | 0.11 | 0.06 | 0.33 | 1.81 | 0.08 | ||||||
| EOT | −0.12 | 0.04 | −0.47 | −3.04 | <.01 | ||||||
Notes: < .05; <.01; Toronto Alexithymia Scale-20: DIF = Difficulty Identifying Feelings, DDF = Difficulty Describing Feelings, EOT = Externally Oriented Thinking.
Hierarchical regression produced a significant model in which delayed memory was predicted by age and sex in Step 1, with EOT (only) adding significant variance in Step 2 (see Table 9). Anxiety and depression, not correlated with memory, provided no prediction when included (ps>.31). Addition of the age-X-EOT and sex-X-EOT interaction terms also produced no significant prediction (ps>.09); age and EOT remained significant. The EOT contribution was particularly notable when examined solely in older adults (while sex lost significance; see Table 9). Indeed, restricted to older adults, simple bivariate correlations of immediate and delayed recall were significant with EOT (but not DIF or DDF; n=42, StoryIR=−.353, p=.022, StoryDR=−.395, p=.010).
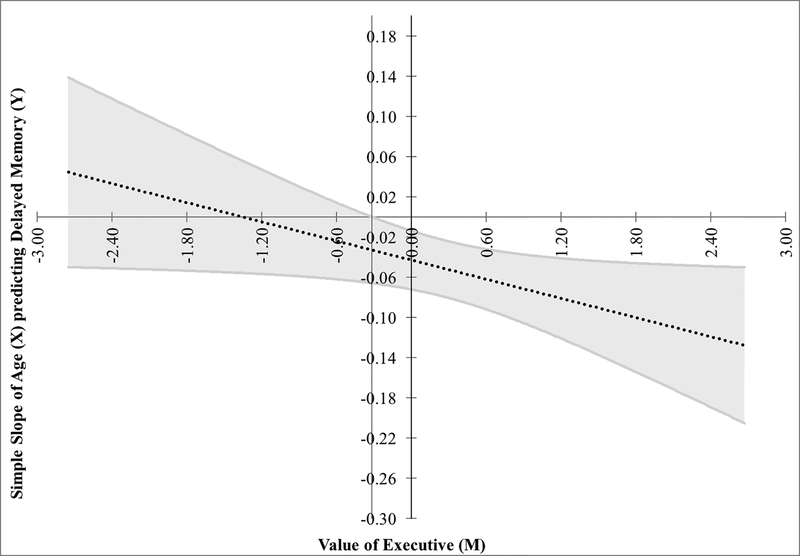
Given the expected correlations between memory and EF, and amongst age, EF and memory, follow-up analyses were conducted to examine potential interactions. EF moderated the relationship between age and memory (R2=.28, p<.001, interaction p=.04). That is, memory showed little variance in young adults and EF did not distinguish it, but the greater variance in memory of older subjects showed that those with poorer EF were particularly impaired (see Figure 2). We further interrogated whether alexithymia, specifically EOT, contributed to this model. The age-X-EOT, EF-X-EOT, and age-X-EF-X-EOT interaction terms were added in successive subsequent steps. No interactions were significant prior to the final step. The final model remained significant (R2=.37, p<.001) with memory predicted by age (B=−.05, p=.003), EF (B=−.79, p=.06), and the 3-way interaction (B=−.81, p=.04; R2change=.04). Figure 3 demonstrates that although memory was poorer at advanced age, poorer executive functions were predictive of poor memory specifically in those who also had high EOT.
Figure 2.
Experiment 3 data plotted using the Johnson-Neyman technique for moderation. The simple slope of age (X) predicting delayed narrative recall (Y) is plotted against the moderator (M), executive functioning (EF). These are shown with the slope (dotted line) and 95% confidence interval (grey band). The vertical black line (left of the Y axis) indicates the significance region where the slope crosses zero (−0.317); greater values, where the grey band does not include 0, were statistically significant. That is, EF from this point and greater (i.e., poorer performance) significantly moderated the relationship between age and memory. Thus, although memory was poorer in older than young adulthood, poor EF was highly detrimental to memory, especially in older age.
Figure 3.
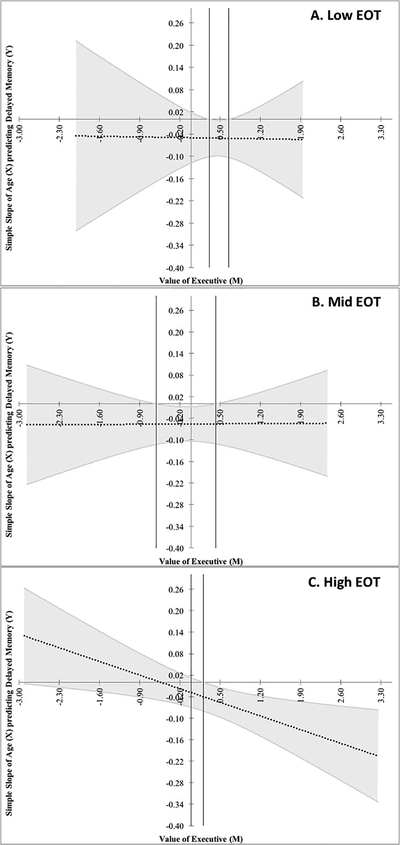
Experiment 3 data, plotted using the Johnson-Neyman procedure, to examine whether alexithymia (via Externally Oriented Thinking (EOT)) moderated the model shown in Figure 2 (i.e., moderated moderation). The simple slope (dotted line; grey band=95% confidence interval (CI)) of age (X) predicting delayed narrative recall (Y) is plotted against the moderator (M), executive functioning (EF, positive score=poorer EF), split by EOT tertile: A. Low EOT (≤ 15), B. Mid EOT (16–19), and C. High EOT (≥ 20). Vertical black lines show significance regions where the slope crosses zero and beyond which, where CI does not include 0, constitutes statistically significant moderation (A: ≥0.32, ≤0.65; B: ≥−0.61, ≤0.43; C: ≥0.21). However, only panel C showed a significant slope for moderation. These plots confirm the 3-way interaction (i.e., moderated moderation) indicating that although EF moderated the relationship between age and memory (see Figure 2), EOT moderated that moderation. That is, poor EF predicted poor memory with greater age, but it did so specifically in those who also had high EOT (panel C), indicating that alexithymia is a risk factor for cognitive dysfunction in older age.
Experiment 3 supports Experiments 1 and 2, indicating alexithymia is associated with poorer EF via DIF and poorer memory via EOT. Furthermore, poor EF substantively contributed to poorer memory, which was specific to those with high EOT. Thus, memory deficits for neutral memoranda were, at least in part, due to generally poorer EF in older adults, and alexithymia. As Experiments 2 and 3 showed that high DIF contributes specifically to executive dysfunction, these results suggest that elevated DIF and EOT may both be risk factors for age-related cognitive decline.
General Discussion
Three independent experiments suggest alexithymia contributes to generally poorer memory and EF in younger and older adults. Specifically, one-hour delayed memory for neutral words was poorer in young adults with high EOT (Exp. 1), and young and older adults with HA via DIF had poorer EF, as measured by a composite of non-emotive standardized tests (Exp. 2). Furthermore, DIF had greater prediction of EF at older age. In Experiment 3, young and older adults with high EOT had poorer 30-min delayed memory for neutral narratives, and those with higher DIF had poorer EF. Moreover, poorer memory was explained by those with poor EF, and specifically those who also had high EOT (see Figure 3).
Importantly, poorer memory and EF were associated with alexithymia using neutral stimuli. This is novel; prior studies show poorer memory only for emotive material (DiStefano & Koven, 2012; Dressaire et al., 2015; Luminet et al., 2006b; Meltzer & Nielson, 2010; Vermeulen & Luminet, 2009) or contexts (Nielson & Meltzer, 2009; Vermeulen et al., 2010). Stimulus relevance may partially explain prior studies. Attentional resources are typically directed toward salient information, which subsequently modulates memory (Mather & Sutherland, 2011; Nielson & Correro, 2017). Alexithymia is fundamentally a difficulty of processing emotive content (Vermeulen et al., 2006). Thus, emotion words receive less boost from salience (Luminet et al., 2006b), making encoding and retrieval of emotive information particularly difficult (Vermeulen et al., 2018). Conversely, alexithymia is frequently associated with functional somatic symptoms (Taylor, Parker, Bagby, & Acklin, 1992), making illness-related information more salient and better remembered (Meltzer & Nielson, 2010). Salience of neutral memoranda in HA is unclear. Nevertheless, EOT predicted worse memory for neutral word lists and stories, suggesting salience effects may be additive to a generalized memory effects in alexithymia, rather than exclusive. Prior studies may have missed this due to small sample sizes and examining neutral material in emotional contexts.
EOT was influential to neutral memory (Experiments 1, 3). Although DIF also predicted memory (Exp. 1), EOT mediated that relationship. These findings extend one prior aging study (Onor et al., 2010) to encompass a broader range of age and context. Prior studies indicated DIF contributed to poorer emotional memory (Luminet et al., 2006b; Vermeulen & Luminet, 2009), while EOT contributed to better neutral memory (Meltzer & Nielson, 2010; Vermeulen & Luminet, 2009). Yet, salience may have influenced those findings (Ferre, Fraga, Comesana, & Sanchez-Casas, 2015), consistent with the emotion processing deficits typifying alexithymia (Luminet et al., 2006b), as these studies examined neutral materials alongside emotional material or in emotive contexts. Some suggest memory effects in alexithymia may depend on separate emotive (i.e., DIF) and cognitive (i.e., EOT) mechanisms and brain regions that, respectively, influence encoding and retrieval (Vermeulen & Luminet, 2009). With non-emotive memoranda, DIF and DDF would play lesser roles, while an externally oriented style may contribute to difficulty limiting distraction during encoding and retrieval. Indeed, for words and stories, high EOT was detrimental to memory performance, across variations in age, sample size, retention interval and memory type (i.e., recall, recognition).
The words used in Experiment 1 were highly imageable, which can assist learning. As EOT can impede utilisation of such encoding resources, poor encoding might be responsible for the memory results. Nevertheless, delayed memory was better predicted by alexithymia than immediate memory. Some also suggest alexithymia influences decision criteria (Jacob & Hautekeete, 1998) or promotes guessing (Vermeulen & Luminet, 2009). Yet, the results were specific to sensitivity rather than guessing. These issues warrant further investigation, but the findings indicate that memory influence of alexithymia extends beyond encoding.
DIF was particularly impactful on EF, supporting previous demonstrations of verbal fluency deficits in alexithymia (e.g., Henry et al., 2006) and more general EF findings undifferentiated by TAS-20 subscale (Koven & Thomas, 2010; Wood & Williams, 2007; Zhang et al., 2011). Other studies, especially those emphasizing EF via fluency, instead reported DDF correlated with EF (Correro et al., 2016; Santorelli & Ready, 2015), which we did not replicate. The EF variable in the current study was a composite of EF tasks including attentional control, sequencing, switching, semantic control, and processing speed. While DIF and DDF are highly correlated, our factor perhaps biased findings toward the ability to interpret internal experience (DIF) rather than capacity to effectively communicate emotion state (DDF), as fluency alone might tap. Overall, the present experiments extend previous findings suggesting a generalized EF deficit in alexithymia. Moreover, Experiments 2 and 3 suggest that the contribution of alexithymia, specifically DIF, to executive dysfunction increases with advancing age, highlighting DIF as a risk factor for age-related cognitive decline.
Higher-order cognitive processes are essential to allocating cognitive resources toward other complex abilities, such as memory (Duff et al., 2005), perception and interpretation of cognitive, emotional (i.e., mentalisation, Aboulafia-Brakha, Christe, Martory, & Annoni, 2011), and bodily states (i.e., interoception, Menon & Uddin, 2010). Both mentalisation and interoception require internal awareness to interpret ones’ own state and what others may be experiencing. Indeed, the social and emotional dysfunction common in alexithymia is often characterized by difficulty with mentalising (Moriguchi et al., 2006; Wastell & Taylor, 2002); interoception (Murphy, Catmur, & Bird, 2017); an external cognitive style; and difficulty with abstraction, which is essential for emotion identification and regulation (Rinaldi, Radian, Rossignol, Arachchige, & Lefebvre, 2017; Wotschack & Klann-Delius, 2013). Internal representations are not readily accessible in HA (Rinaldi et al., 2017), leading to ineffective application of a ‘primitive’ and physiological level of emotional awareness (Bermond, Vorst, & Moormann, 2006; Kano & Fukudo, 2013; Lane et al., 1996). Similarly, poorer EF is associated with DIF—a feature at the intersection of bottom-up emotional awareness and top-down interpretation of social and emotive content and situations (Bar-On, Tranel, Denburg, & Bechara, 2003; Frawley & Smith, 2001). This appears to describe alexithymia, where instrumental relationships are manageable but more expressive and complex relationships are impeded (Wastell & Taylor, 2002). Our findings further reinforce these characterizations.
Although alexithymia did not correlate with age in our full samples, it did significantly correlate with age within older samples, consistent with previous research (Gunzelmann, Kupfer, & Brahler, 2002; Mattila et al., 2006; Salminen, Saarijarvi, Aarela, Toikka, & Kauhanen, 1999). Indeed, some suggest alexithymia may be better understood as a reflection of generalized neurocognitive functioning (Messina, Beadle, & Paradiso, 2014; Sturm & Levenson, 2011) and that it may reflect a neuropsychiatric consequence of normal aging (Santorelli & Ready, 2015). Despite deficits in cognitive control that are typical with aging (e.g., Gutchess, 2019; Nielson, Langenecker, & Garavan, 2002), our results support previous findings of exacerbated deficits in alexithymia. Indeed, young and older adults with high EOT have shown difficulty intentionally inhibiting the recall of negative information, suggesting alexithymia may contribute more to impairment in cognitive control than does age (Dressaire et al., 2015). In the current study, age and alexithymia (DIF) both predicted EF, with some indication that DIF is increasingly impactful at older age, and in Experiment 3, poorer memory in older age was characterized by those with poor EF and high EOT. Thus, alexithymia added significant predictive value beyond and in interaction with aging effects to understanding memory performance in older adults. This complex interplay of age, EF, memory and alexithymia is consistent with the accumulating neuroimaging studies in alexithymia showing reduced grey matter volume and deficits in interhemispheric and cortical-subcortical transfer and cortical networks (see Goerlich & Aleman, 2018).
This study had limitations. The samples were large compared with most relevant studies, but smaller in Experiment 3, which could affect generalizability. This study reflected a secondary analysis of previous experiments; differences across samples (e.g., measures, tasks, exclusion/inclusion criteria, retention interval, what filled the interval) and representation of middle adulthood could be improved upon in future work. Our EF factor results are generally consistent with verbal fluency (Henry et al., 2006; Santorelli & Ready, 2015) and attentional control (Wood & Williams, 2007; Zhang et al., 2011) deficits in HA, but future work disentangling the role of discrete executive abilities in alexithymia is important. As EOT has low IC, future memory studies might consider the items of this subscale in detail. Finally, examination of the possible neural foundations of alexithymia, as well as interventions directed at enhancing cognition or reducing alexithymia, might help to clarify directionality of effect between cognition and alexithymia.
Conclusion
Across three independent experiments, alexithymia was associated with poorer delayed memory for neutral memoranda and poorer general EF. An external cognitive style (i.e., EOT) was responsible for the role of alexithymia in memory, and difficulty with emotion perception (i.e., DIF) was responsible for executive dysfunction. Critically, poor EF contributed to poorer memory at older age, and EOT further contributed such that those who had high EOT and poorer executive ability had the poorest memory at advanced age. EOT likely precludes the use of internal cognitive control that facilitates memory processes (Dressaire et al., 2015), suggesting memory and executive dysfunction in alexithymia may be understood by the inefficient use of or ineffective access to internal awareness (Moriguchi & Komaki, 2013). Based on the present results, we posit that executive functions are generally impaired in those with higher alexithymia via DIF, and the tendency to view the world externally (i.e., EOT) hinders internal monitoring and memory functions, even in non-emotive contexts. Therefore, alexithymia is a potentially substantive contributor to age-related cognitive dysfunction.
Acknowledgements
The work was supported by a grant from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources and the National Center for Advancing Translational Science (8UL1TR000055), a Way-Klingler Sabbatical Fellowship award from Marquette University, a National Science Foundation Graduate Research Fellowship (#1452781) and grants from the Scientific Research Network on Decision Neuroscience and Aging (Stanford University Center on Longevity), National Center for Advancing Translational Sciences, and the National Institutes of Health (UL1TR001436 and TL1TR001437). For assistance with various aspects of these experiments, the authors gratefully acknowledge the contributions of Drs. Kathleen Hazlett Elverman, Shaun English, Christina Figueroa, Stephanie Potts, and Katherine Reiter; and David Amy, Crystal Becker, Jessica Burkard, Megan Fabisch, Emily Gaber, Zachary Grese, Abigail Helbling, Joshua Krueger, Sarah Lentes, Riley Marinelli, Cara Macchia, Carolyn Madry, David Marra, Marie Mejaki, Emma Murry, Holly Robertson, Stephanie Ocwieja, Kara Pierce, Angela Preston, Stephenie, Quirke, Kristen Skells, Olivia Speeter, Megan Spoo, Aubrey Tschanz, Janel Wasisco, and Alex Zurek. The authors have no conflicts of interest to report.
Footnotes
Multiple measures collected for these protocols (i.e., original purposes, hypothesis) masking are not shown. The corresponding author can provide a complete list. Validated test ordering was used to avoid contamination (e.g., no verbal/memory tests during memory retention). All subjects with valid data were included. All procedures were approved by the Institutional Review Board (IRB).
Normal range was exceeded if: both depression and anxiety T-scores > 63 (n=15 Experiment 1, n=15, Experiment 2, n=9 (7 young)); or BDI > 13 and/or BAI > 15 (mild) (Experiment 3, n=5, all young). Removal of these subjects from models did not alter study results.
References
- Aboulafia-Brakha T, Christe B, Martory MD, & Annoni JM (2011). Theory of mind tasks and executive functions: a systematic review of group studies in neurology. J Neuropsychol, 5(Pt 1), 39–55. doi: 10.1348/174866410X533660 [DOI] [PubMed] [Google Scholar]
- Aftanas LI, Varlamov AA, Reva NV, & Pavlov SV (2003). Disruption of early event-related theta synchronization of human EEG in alexithymics viewing affective pictures. Neurosci Lett, 340(1), 57–60. doi: 10.1016/S0304-3940(03)00070-3 [DOI] [PubMed] [Google Scholar]
- Bagby RM, Parker JD, & Taylor GJ (1994). The twenty-item Toronto Alexithymia Scale--I. Item selection and cross-validation of the factor structure. J Psychosom Res, 38(1), 23–32. doi: 10.1016/0022-3999(94)90005-1 [DOI] [PubMed] [Google Scholar]
- Bagby RM, Taylor GJ, & Parker JD (1994). The Twenty-item Toronto Alexithymia Scale--II. Convergent, discriminant, and concurrent validity. J Psychosom Res, 38(1), 33–40. doi: 10.1016/0022-3999(94)90006-X [DOI] [PubMed] [Google Scholar]
- Bar-On R, Tranel D, Denburg NL, & Bechara A (2003). Exploring the neurological substrate of emotional and social intelligence. Brain, 126(Pt 8), 1790–1800. doi: 10.1093/brain/awg177 [DOI] [PubMed] [Google Scholar]
- Beck AT, & Steer RA (1990). Manual for the Beck Anxiety Inventory. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Beck Depression Inventory-II (BDI-II). San Antonio, TX: Psychological Corporation. [Google Scholar]
- Bermond B, Vorst HC, & Moormann PP (2006). Cognitive neuropsychology of alexithymia: implications for personality typology. Cogn Neuropsychiatry, 11(3), 332–360. doi: 10.1080/13546800500368607 [DOI] [PubMed] [Google Scholar]
- Carden SW, Holtzman NS, & Strube MJ (2017). CAHOST: An excel workbook for facilitating the Johnson-Neyman technique for two-way interactions in multiple regression. Frontiers in Psychology, 8(1293), 1–7. doi: 10.3389/fpsyg.2017.01293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton RA, Landau S, Schiavone F, Barrick TR, Clark CA, Markus HS, & Morris RG (2008). A structural equation modeling investigation of age-related variance in executive function and DTI measured white matter damage. Neurobiol Aging, 29(10), 1547–1555. doi: 10.1016/j.neurobiolaging.2007.03.017 [DOI] [PubMed] [Google Scholar]
- Correro AN, Marra DE, Reiter K, Byers SJ, & Nielson KA (2016). Executive functioning and verbal memory are predictive of alexithymia across the lifespan [Abstract]. Clin Neuropsychol, 30(3), 425. [Google Scholar]
- da Silva AN, Vasco AB, & Watson JC (2017). Alexithymia and Emotional Processing: A Mediation Model. J Clin Psychol, 73(9), 1196–1205. doi: 10.1002/jclp.22422 [DOI] [PubMed] [Google Scholar]
- Derogatis LR, & Melisaratos N (1983). The Brief Symptom Inventory: an introductory report. Psychological Medicine, 13, 595–605. [PubMed] [Google Scholar]
- Diamond A (2013). Executive Functions. Annual Review of Psychology, Vol 64, 64, 135–168. doi: 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiStefano RA, & Koven NS (2012). Dysfunctional emotion processing may explain visual memory deficits in alexithymia. Pers Indiv Differ, 52(5), 611–615. doi: 10.1016/j.paid.2011.12.007 [DOI] [Google Scholar]
- Dressaire D, Stone CB, Nielson KA, Guerdoux E, Martin S, Brouillet D, & Luminet O (2015). Alexithymia impairs the cognitive control of negative material while facilitating the recall of neutral material in both younger and older adults. Cogn Emot, 29(3), 442–459. doi: 10.1080/02699931.2014.919898 [DOI] [PubMed] [Google Scholar]
- Duff K, Schoenberg MR, Scott JG, & Adams RL (2005). The relationship between executive functioning and verbal and visual learning and memory. Arch Clin Neuropsychol, 20(1), 111–122. doi: 10.1016/j.acn.2004.03.003 [DOI] [PubMed] [Google Scholar]
- Ferre P, Fraga I, Comesana M, & Sanchez-Casas R (2015). Memory for emotional words: The role of semantic relatedness, encoding task and affective valence. Cogn Emot, 29(8), 1401–1410. doi: 10.1080/02699931.2014.982515 [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Frawley W, & Smith RN (2001). A processing theory of alexithymia. Cognitive Systems Research, 2(3), 189–206. doi: 10.1016/S1389-0417(01)00029-8 [DOI] [Google Scholar]
- Goerlich KS, & Aleman A (2018). Neuroimaging studies of alexithymia. In Luminet O, Bagby RM, & Taylor GJ(Eds.), Alexithymia: Advances in research, theory and clinical practice (pp. 207–249). Cambridge, UK: Cambridge University Press. [Google Scholar]
- Gunzelmann T, Kupfer J, & Brahler E (2002). Alexithymia in the elderly general population. Compr Psychiatry, 43(1), 74–80. doi: 10.1053/comp.2002.29855 [DOI] [PubMed] [Google Scholar]
- Gutchess A (2019). Cognitive and Social Neuroscience of Aging. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Hayes AF (2018). Introduction to mediation, moderation, and conditional process analysis : a regression-based approach (Second edition. ed.). New York: Guilford Press. [Google Scholar]
- Henry JD, Phillips LH, Crawford JR, Theodorou G, & Summers F (2006). Cognitive and psychosocial correlates of alexithymia following traumatic brain injury. Neuropsychologia, 44(1), 62–72. doi: 10.1016/j.neuropsychologia.2005.04.011 [DOI] [PubMed] [Google Scholar]
- Hill BD, Alosco M, Bauer L, & Tremont G (2012). The relation of executive functioning to CVLT-II learning, memory, and process indexes. Appl Neuropsychol Adult, 19(3), 198–206. doi: 10.1080/09084282.2011.643960 [DOI] [PubMed] [Google Scholar]
- Jacob S, & Hautekeete M (1998). Alexithymia and memory: a more rigorous criterion for acceptance in recognition tasks? Encephale-Revue De Psychiatrie Clinique Biologique Et Therapeutique, 24(3), 199–204. [PubMed] [Google Scholar]
- Kano M, & Fukudo S (2013). The alexithymic brain: the neural pathways linking alexithymia to physical disorders. Biopsychosoc Med, 7(1), 1. doi: 10.1186/1751-0759-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koven NS, & Thomas W (2010). Mapping facets of alexithymia to executive dysfunction in daily life. Pers Indiv Differ, 49, 24–28. doi: 10.1016/j.paid.2010.02.034 [DOI] [Google Scholar]
- Lamberty GJ, & Holt CS (1995). Evidence for a verbal deficit in alexithymia. J Neuropsychiatry Clin Neurosci, 7(3), 320–324. doi: 10.1176/jnp.7.3.320 [DOI] [PubMed] [Google Scholar]
- Lane RD, Sechrest L, Reidel R, Weldon V, Kaszniak A, & Schwartz GE (1996). Impaired verbal and nonverbal emotion recognition in alexithymia. Psychosom Med, 58(3), 203–210. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW, & Fischer JS (2004). Neuropsychological assessment. U.S.A.: Oxford University Press. [Google Scholar]
- Luminet O, Vermeulen N, Demaret C, Taylor GJ, & Bagby RM (2006a). Alexithymia and levels of processing: Evidence for an overall deficit in remembering emotion words. Journal of Research in Personality, 40(5), 713–733. doi: 10.1016/j.jrp.2005.09.001 [DOI] [Google Scholar]
- Luminet O, Vermeulen N, Demaret C, Taylor GJ, & Bagby RM (2006b). Alexithymia and levels of processing: Evidence for an overall deficit in remembering emotion words. J Res Pers, 40(5), 713–733. doi: 10.1016/j.jrp.2005.09.001 [DOI] [Google Scholar]
- Mather M, & Sutherland MR (2011). Arousal-Biased Competition in Perception and Memory. Perspect Psychol Sci, 6(2), 114–133. doi: 10.1177/1745691611400234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila AK, Salminen JK, Nummi T, & Joukamaa M (2006). Age is strongly associated with alexithymia in the general population. J Psychosom Res, 61(5), 629–635. doi: 10.1016/j.jpsychores.2006.04.013 [DOI] [PubMed] [Google Scholar]
- Meltzer MA, & Nielson KA (2010). Memory for emotionally provocative words in alexithymia: a role for stimulus relevance. Conscious Cogn, 19(4), 1062–1068. doi: 10.1016/j.concog.2010.05.008 [DOI] [PubMed] [Google Scholar]
- Menon V, & Uddin LQ (2010). Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct, 214(5–6), 655–667. doi: 10.1007/s00429-010-0262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina A, Beadle JN, & Paradiso S (2014). Towards a classification of alexithymia: Primary, secondary and organic. Journal of Psychopathology, 20(1), 38–49. [Google Scholar]
- Moriguchi Y, & Komaki G (2013). Neuroimaging studies of alexithymia: physical, affective, and social perspectives. Biopsychosoc Med, 7(1), 8. doi: 10.1186/1751-0759-7-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y, Ohnishi T, Lane RD, Maeda M, Mori T, Nemoto K, … Komaki G (2006). Impaired self-awareness and theory of mind: an fMRI study of mentalizing in alexithymia. Neuroimage, 32(3), 1472–1482. doi: 10.1016/j.neuroimage.2006.04.186 [DOI] [PubMed] [Google Scholar]
- Murphy J, Catmur C, & Bird G (2017). Alexithymia Is Associated With a Multidomain, Multidimensional Failure of Interoception: Evidence From Novel Tests. J Exp Psychol Gen. doi: 10.1037/xge0000366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemiah JC (1977). Alexithymia. Theoretical considerations. Psychother Psychosom, 28(1–4), 199–206. doi: 10.1159/000287064 [DOI] [PubMed] [Google Scholar]
- Nielson KA, & Correro AN 2nd. (2017). Post-learning arousal enhances veridical memory and reduces false memory in the Deese-Roediger-McDermott paradigm. Neurobiol Learn Mem, 144, 198–207. doi: 10.1016/j.nlm.2017.07.009 [DOI] [PubMed] [Google Scholar]
- Nielson KA, Langenecker SA, & Garavan H (2002). Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychol Aging, 17(1), 56–71. doi: 10.1037/0882-7974.17.1.56 [DOI] [PubMed] [Google Scholar]
- Nielson KA, & Meltzer MA (2009). Modulation of long-term memory by arousal in alexithymia: the role of interpretation. Conscious Cogn, 18(3), 786–793. doi: 10.1016/j.concog.2009.06.001 [DOI] [PubMed] [Google Scholar]
- Nielson KA, Yee D, & Erickson KI (2005). Memory enhancement by a semantically unrelated emotional arousal source induced after learning. Neurobiology of Learning and Memory, 84, 49–56. doi: 10.1016/j.nlm.2005.04.001 [DOI] [PubMed] [Google Scholar]
- Onor M, Trevisiol M, Spano M, Aguglia E, & Paradiso S (2010). Alexithymia and aging: a neuropsychological perspective. J Nerv Ment Dis, 198(12), 891–895. doi: 10.1097/NMD.0b013e3181fe743e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paivio A, Yuille JC, & Madigan SA (1968). Concreteness, imagery, and meaningfulness values for 925 nouns. Journal of Experimental Psychology, 76(Suppl.), 1–25. doi: 10.1037/h0025327 [DOI] [PubMed] [Google Scholar]
- Parker JD, Taylor GJ, & Bagby RM (2003). The 20-Item Toronto Alexithymia Scale. III. Reliability and factorial validity in a community population. J Psychosom Res, 55(3), 269–275. doi: 10.1016/S0022-3999(02)00578-0 [DOI] [PubMed] [Google Scholar]
- Reitan RM (1955). The relation of the trail making test to organic brain damage. J Consult Psychol, 19(5), 393–394. [DOI] [PubMed] [Google Scholar]
- Rinaldi R, Radian V, Rossignol M, Arachchige KGK, & Lefebvre L (2017). Thinking About One’s Feelings Association Between Alexithymia and Cognitive Styles in a Nonclinical Population. Journal of Nervous and Mental Disease, 205(10), 812–815. doi: 10.1097/Nmd.0000000000000721 [DOI] [PubMed] [Google Scholar]
- Rubin DC (2006). The Basic-Systems Model of Episodic Memory. Perspect Psychol Sci, 1(4), 277–311. doi: 10.1111/j.1745-6916.2006.00017.x [DOI] [PubMed] [Google Scholar]
- Salminen JK, Saarijarvi S, Aarela E, Toikka T, & Kauhanen J (1999). Prevalence of alexithymia and its association with sociodemographic variables in the general population of Finland. J Psychosom Res, 46(1), 75–82. doi: 10.1016/S0022-3999(98)00053-1 [DOI] [PubMed] [Google Scholar]
- Santorelli GD, & Ready RE (2015). Alexithymia and Executive Function in Younger and Older Adults. Clin Neuropsychol, 29(7), 938–955. doi: 10.1080/13854046.2015.1123296 [DOI] [PubMed] [Google Scholar]
- Sifneos PE (1973). The prevalence of ‘alexithymic’ characteristics in psychosomatic patients. Psychother Psychosom, 22(2), 255–262. doi: 10.1159/000286529 [DOI] [PubMed] [Google Scholar]
- Smith A (1991). Symbol Digit Modalities Test. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Soper DS (2019). A-priori Sample Size Calculator for Multiple Regression [Software]. Retrieved from http://www.danielsoper.com/statcalc
- Stanislaw H, & Todorov N (1999). Calculation of signal detection theory measures. Behavior research methods, instruments, & computers, 31(1), 137–149. [DOI] [PubMed] [Google Scholar]
- Sturm VE, & Levenson RW (2011). Alexithymia in neurodegenerative disease. Neurocase, 17(3), 242–250. doi: 10.1080/13554794.2010.532503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suslow T, Kersting A, & Arolt V (2003). Alexithymia and incidental learning of emotional words. Psychol Rep, 93(3 Pt 2), 1003–1012. doi: 10.2466/pr0.2003.93.3f.1003 [DOI] [PubMed] [Google Scholar]
- Swart M, Kortekaas R, & Aleman A (2009). Dealing with feelings: characterization of trait alexithymia on emotion regulation strategies and cognitive-emotional processing. PLoS One, 4(6), e5751. doi: 10.1371/journal.pone.0005751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GJ (2000). Recent developments in alexithymia theory and research. Can J Psychiatry, 45(2), 134–142. doi: 10.1177/070674370004500203 [DOI] [PubMed] [Google Scholar]
- Taylor GJ, Bagby RM, & Luminet O (2000). Assessment of alexithymia: Self-report and observer-rated measures. In Parker JDA & Bar-On R (Eds.), The Handbook of Emotional Intelligence (pp. 301–319). San Francisco, CA: Jossey Bass. [Google Scholar]
- Taylor GJ, Bagby RM, & Parker JD (2003). The 20-Item Toronto Alexithymia Scale. IV. Reliability and factorial validity in different languages and cultures. J Psychosom Res, 55(3), 277–283. doi: 10.1016/S0022-3999(02)00601-3 [DOI] [PubMed] [Google Scholar]
- Taylor GJ, Parker JD, Bagby RM, & Acklin MW (1992). Alexithymia and somatic complaints in psychiatric out-patients. J Psychosom Res, 36(5), 417–424. doi: 10.1016/0022-3999(92)90002-J [DOI] [PubMed] [Google Scholar]
- van der Velde J, Gromann PM, Swart M, Wiersma D, de Haan L, Bruggeman R, … Aleman A (2015). Alexithymia influences brain activation during emotion perception but not regulation. Soc Cogn Affect Neurosci, 10(2), 285–293. doi: 10.1093/scan/nsu056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen N, Domachowska I, & Nielson KA (2018). Memory and executive functions in alexithymia. In Luminet O, Bagby RM, & Taylor GJ (Eds.), Alexithymia: Advances in research, theory, and clinical practice (pp. 78–89). Cambridge, United Kingdom: Cambridge University Press. [Google Scholar]
- Vermeulen N, & Luminet O (2009). Alexithymia factors and memory performances for neutral and emotional words. Pers Indiv Differ, 47, 305–309. doi: 10.1016/j.paid.2009.03.018 [DOI] [Google Scholar]
- Vermeulen N, Luminet O, & Corneille O (2006). Alexithymia and the automatic processing of affective information: Evidence from the affective priming paradigm. Cognition & Emotion, 20(1), 64–91. doi: 10.1080/02699930500304654 [DOI] [Google Scholar]
- Vermeulen N, Toussaint J, & Luminet O (2010). The influence of alexithymia and music on the incidental memory for emotion words. Eur J Personality, 24(6), 551–568. doi: 10.1002/per.758 [DOI] [Google Scholar]
- Wang WC, Daselaar SM, & Cabeza R (2017). Episodic memory decline and healthy aging. In Stein J (Ed.), Reference module in neuroscience and biobehavioral psychology. Nijmegen, Nederlands: Radboud University. [Google Scholar]
- Wastell CA, & Taylor AJ (2002). Alexithymic mentalising: Theory of mind and social adaptation. Social Behavior and Personality, 30(2), 141–148. doi: 10.2224/sbp.2002.30.2.141 [DOI] [Google Scholar]
- Wilson B, Cockburn J, Baddeley A, & Hiorns R (1989). The development and validation of a test battery for detecting and monitoring everyday memory problems. Journal of Clinical and Experimental Neuropsychology, 11(6), 855–870. doi: 10.1080/01688638908400940 [DOI] [PubMed] [Google Scholar]
- Wood RL, & Williams C (2007). Neuropsychological correlates of organic alexithymia. J Int Neuropsychol Soc, 13(3), 471–479. doi: 10.1017/S1355617707070518 [DOI] [PubMed] [Google Scholar]
- Wotschack C, & Klann-Delius G (2013). Alexithymia and the conceptualization of emotions: A study of language use and semantic knowledge. Journal of Research in Personality, 47(5), 514–523. doi: 10.1016/j.jrp.2013.01.011 [DOI] [Google Scholar]
- Zhang L, Ye R, Yu F, Cao Z, Zhu C, Cai Z, … Wang K (2012). How does emotional context modulate response inhibition in alexithymia: electrophysiological evidence from an ERP study. PLoS One, 7(12), e51110. doi: 10.1371/journal.pone.0051110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhu C, Ye R, Cao Z, Tian Y, Yang P, … Wang K (2011). Impairment of conflict processing in alexithymic individuals. Neurosci Lett, 504(3), 261–264. doi: 10.1016/j.neulet.2011.09.043 [DOI] [PubMed] [Google Scholar]