Figure 2. Regulation of GSK3-β activity.
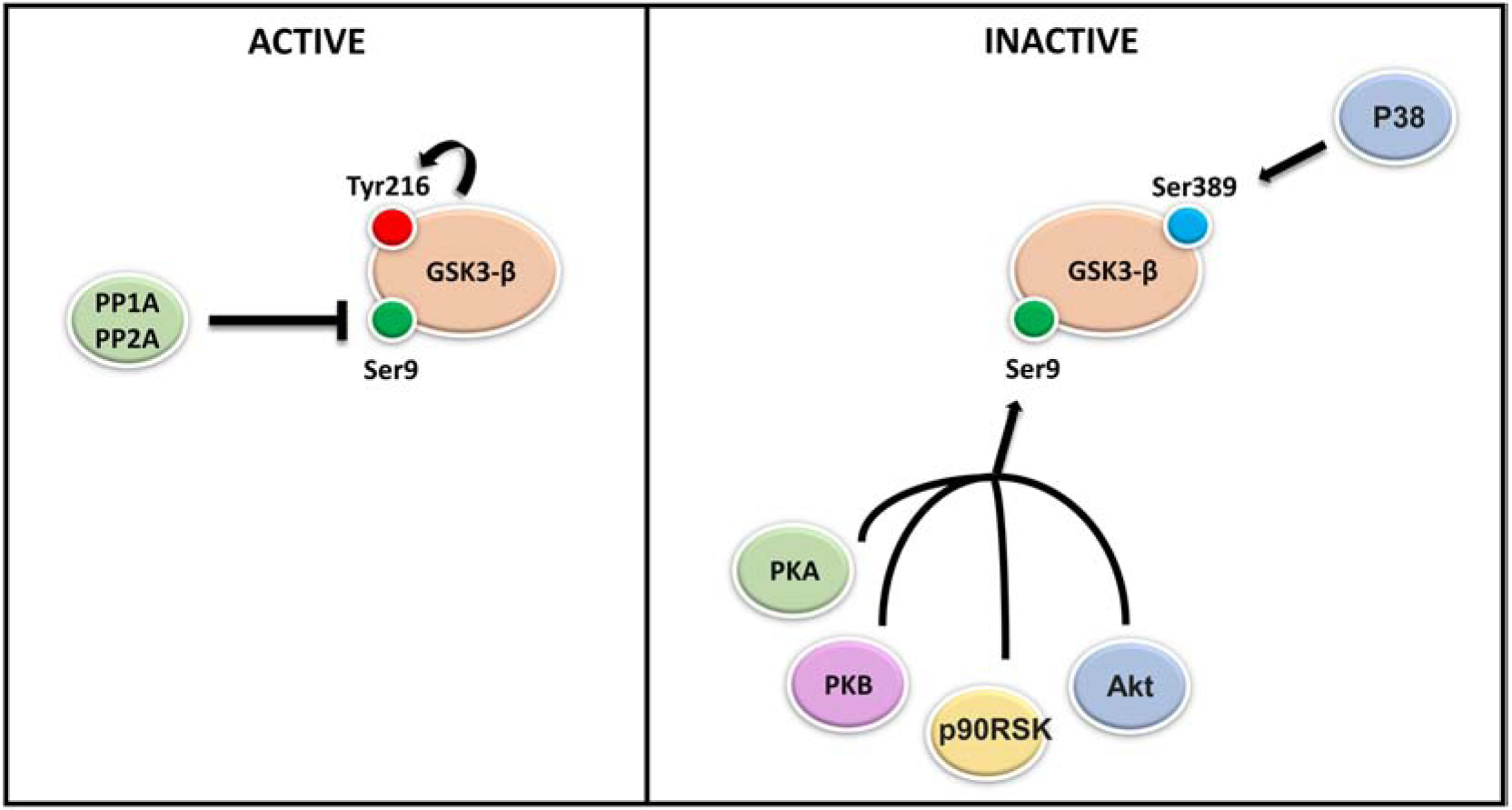
GSK3-β exists in an active and in inactive form depending on its phosphorylation status. Two families of enzymes modulate GSK3-β activity: Serine/Threonine and Tyrosine kinases which attach phosphate groups to GSK3-β and phosphatases which remove phosphate groups from GSK3-β protein in response to external stimuli. In vitro and in vivo evidence have shown that, GSK3-β activation is mediated by auto-phosphorylation on tyrosine-216, while its inhibition is mediated by phosphorylation on serine 9 by several kinases including: AKT, protein kinase A and B (PKA-PKB) and P90RSK. GSK3-β phosphorylation on serine 389 by P38 MAPK kinase instead, has been shown to inhibit GSK3-β. Additionally, the phosphate groups added to GSK3-β during phosphorylation can be removed by protein phosphatase PP2A resulting in GSK3-β re-activation.
