Figure 3. GSK3-β interferes with Aβ and tau metabolism.
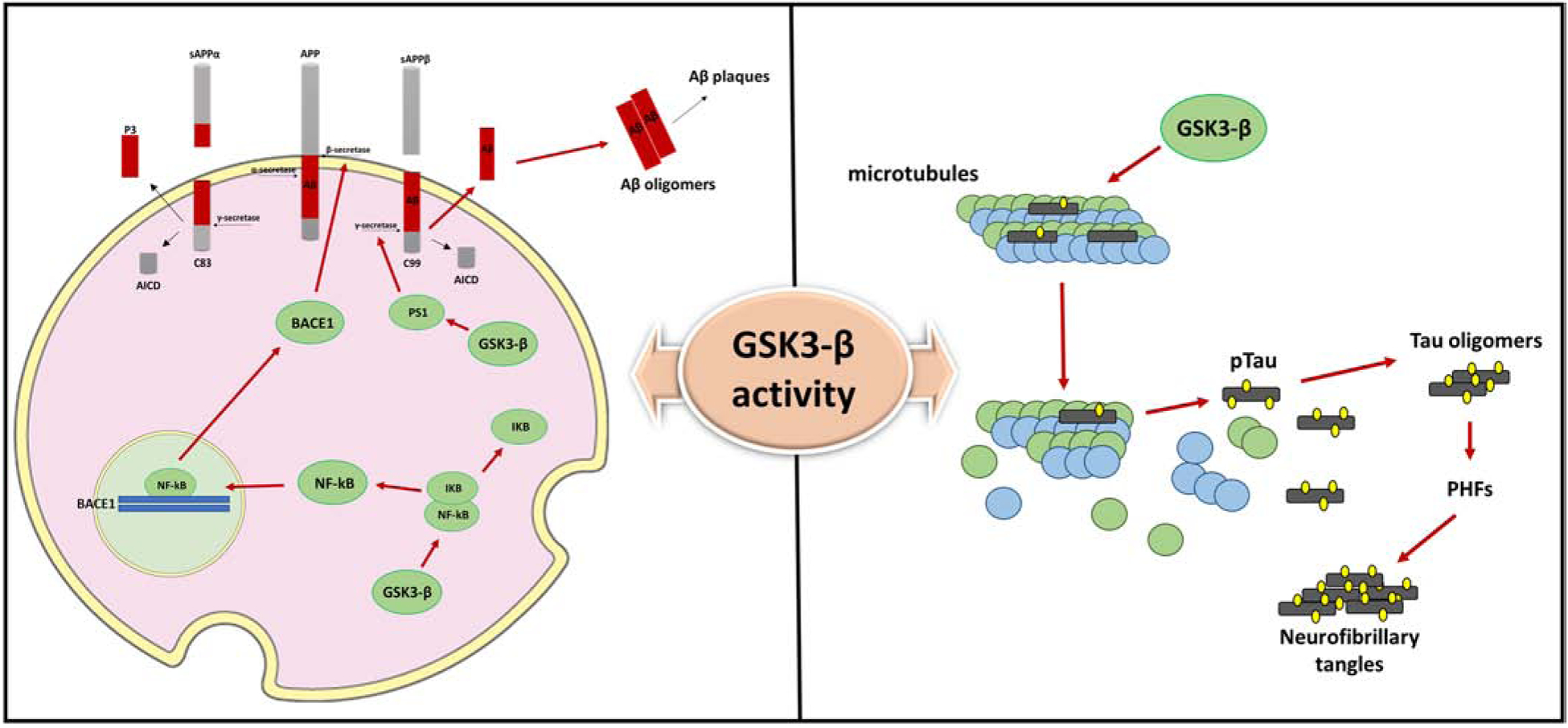
Increased activity of GSK3-β promotes Aβ formation via two distinct mechanisms: GSK3-β induces BACE1 gene expression via upregulation of NFK-β signaling and modulates γ-secretase activity. 1) The human BACE1 promoter region contains two functional NF-kB-binding sites. In vitro and in vivo evidence have shown that GSK3-β activation is involved in NFK-β/p65 nuclear translocation and binding to the BACE1 promoter sites, ultimately resulting in increased BACE1 protein levels and BACE1 mediated-APP processing and Aβ production. 2) GSK3-β modulates γ-secretase activity by direct interaction and regulation of PS1 activity and cellular localization. Upon GSK3-β phosphorylation, PS1 shows lower binding affinity for N-cadherin and reduced cell-surface expression which alter PS1/γ-secretase substrate specificity.
Additionally, GSK3-β is one of the major kinases involved in tau phosphorylation. The addition of a phosphate group on the specific Thr231 residue of tau protein leads to microtubules disassembly and promotes formation of tau oligomers and NFTs contributing to neuronal dysfunction and degeneration.
