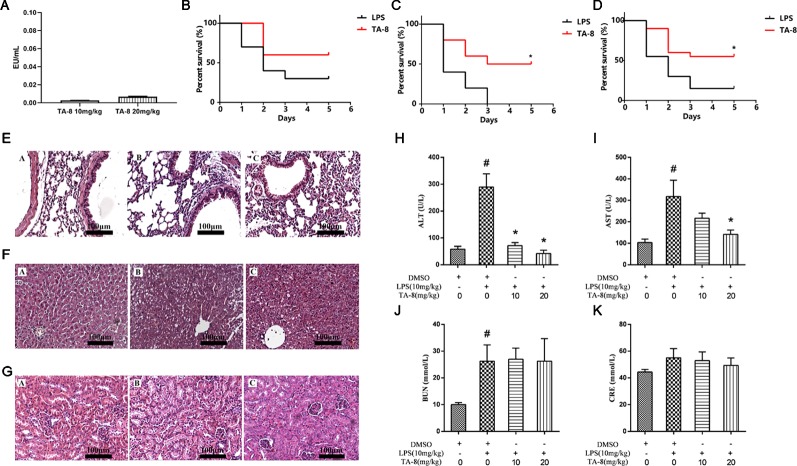
Figure 3.
TA-8 effectively protects mice from lipopolysaccharide (LPS)-induced mortality and reduces lung, liver, and kidney injuries. We examined whether the drug was contaminated with endotoxin. Endotoxin residues have been detected by using limulus amebocyte lysate (LAL) test. The results showed that the endotoxin residue was less than 0.01 EU/ml, which was in compliance with the requirements for injectables (panel A). Ten male mice and ten female mice per group were treated with vehicle only or TA-8 (i.p.) after LPS injection (20 mg/kg, i.p.). Mice were monitored every 8 h for 5 days. Mice treated with TA-8 had significantly higher survival than model mice (female mice panel B; male mice panel C; all mice panel D). C57BL/6 mice were injected with TA-8 or vehicle [dimethyl sulfoxide (DMSO)] 1 h before LPS injection (20 mg/kg, i.p.), tissues of lung, liver and kidney were harvested 20 h after LPS injection. The results show H&E-staining of lung (panel E), liver (panel F), or kidney tissue (panel G) sections from the indicated group (×200). The figure is a representative of three independent experiments. C57BL/6 mice were injected with TA-8 or vehicle (DMSO) 1 h before LPS injection (10 mg/kg, i.p.), and the blood samples were harvested 20 h after LPS injection. The levels of ALT (panel H), AST (panel I), BUN (panel J), and CRE (panel K) were measured (n=3). # P < 0.05 versus the control group; * P < 0.05, versus LPS-stimulated mice.