FIGURE 4.
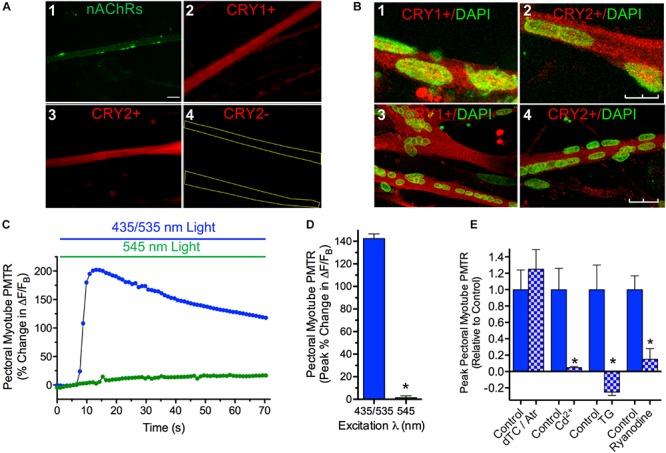
Cytosolic CRY expression in pectoral and iris myotubes; PMT in pectoral myotubes. (A) Striated pectoral myotubes in culture identified by morphology and the presence of nAChR clusters (1) express cytosolic CRY1 (2) and CRY2 (3) proteins when viewed at 40X with epifluorescence microscopy. As described in Methods, AF488-αBgt was used to label nAChRs (green), and anti-CRY pAbs followed by AF536-IgG used to label CRY1 and CRY2 immunoreactivity (red). The CRY immunolabeling was specific since none was seen when primary anti-CRY pAb was omitted (e.g. 4, yellow lines outline myotube borders). Scale bar represents 20 μm and applies to 1–4. (B) Laser scanning confocal imaging confirms extensive cytosolic CRY1 and CRY2 localization in cultured myotubes immunolabeled as in panel A (red) from both iris (1, 2) and pectoral (3, 4) muscle with minimal CRY localization in DAPI-labeled nuclei (green). Images are Z-series projections from 12 to 15 1 μm thick optical sections. Scale bars represents 10 μm in 1 and 2 and 25 μm in 3 and 4. (C) Specific pectoral myotube Ca2+ fluorescence changes expressed as ΔF/FB in response to 435/535 nm dual excitation light exposure (blue bar and circles) as in Figure 4, and lack of response after exposure to 545 nm single wavelength light (green bar and circles). Points reflect % change in ΔF/FB values relative to the value at light onset. (D) Cumulative pectoral myotube PMTRs expressed as peak% change in ΔF/FB (± SEM) in response to dual excitation 435/535 nm light (blue bar; n = 74; N = 19) or single wavelength 545 nm light (green bar; n = 16; N = 7). PMTRs from pectoral myotubes were qualitatively similar to those from iris myotubes but were significantly larger (p < 0.05; see text comparing PMTRs in Figures 4D,3D). Overall, 98% of pectoral myotubes tested displayed PMTRs in response to 435/535 nm dual excitation light. (E) Ca2+ dynamics underlying the PMTR in pectoral myotubes. As with whole iris, AChRs are dispensable since peak myotube PMTRs recorded after incubation with 1 μM ATR and 100 μM dTC (15–30 min) were indistinguishable from controls tested in parallel (n = 11 and 8, respectively, p = 0.5; N = 2 for both). As with whole iris, the myotube PMTR requires external Ca2+ influx and subsequent release from a Ryanodine sensitive intracellular store. Myotube PMT was reduced by 95 ± 1% in n = 4 myotubes incubated for 30 min in RS (normal Ca2+) containing 500 μM Cd2+ to inhibit influx via Ca2+ channels when compared with 6 control myotubes (N = 2 for both). Incubation in RS containing Thapsigargin (TG, 3uM, 1.5 h) reduced the light response by 125 ± 6% in n = 5 test myotubes when compared with 4 time-matched control myotubes (N = 2 for both). RyR mediated Ca2+ release appeared critical since Ryanodine (100 μM, 1.5 h) significantly reduced PMTRs by 85 ± 13% in n = 7 test myotubes when compared with seven time-matched control myotubes (N = 2 for both). Results are expressed as mean peak PMTR (± SEM) for myotubes in test RS conditions (blue/white check columns) relative to control myotubes (blue columns) from the same experiments assayed in normal RS.
