Figure 1. Novel allosteric binding site in class A GPCRs.
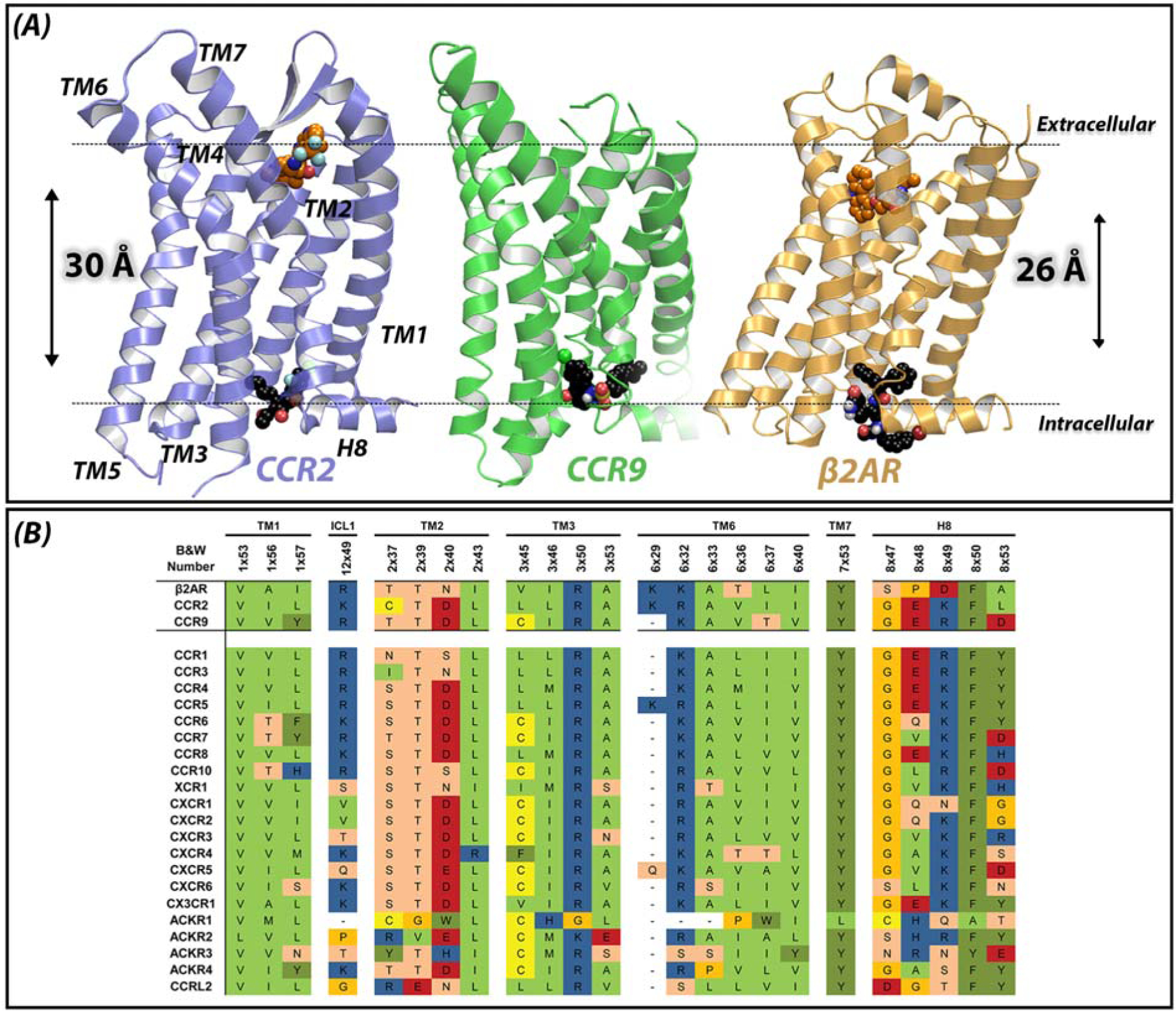
(A) Endogenous ligands bind close to the extracellular region of GPCRs, in the so-called orthosteric binding site. Most of the co-crystallized small molecules also bind in this extracellular region, such as BMS-681 in CCR2 and carazolol in β2AR. Recently, the crystal structures of CCR2 (purple, PDB 5T1A), CCR9 (green, PDB 5LWE) and β2AR (yellow, PDB 4XT1) have revealed an allosteric solvent-exposed binding site, located in the intracellular region of GPCRs, around 30 Å away from the orthosteric binding site. This novel binding site challenges the traditional view of the upper 7TM region of GPCRs as ligand binding domain and the intracellular region as signaling domain only. As shown in the structures, this intracellular binding site can also be targeted by small molecules such as CCR2-RA-[R] in CCR2, vercirnon in CCR9 and 15-PA in β2AR. Dotted lines represent the plane of the membrane. (B) Sequence conservation among chemokine receptors and β2AR, based on the GPCR database (GPCRdb, http://www.gpcrdb.org). Residues shown are residues involved in the intracellular binding site of CCR2, CCR9 and β2AR (upper three rows). Some of these residues have also been found to be important for ligand binding to other class A GPCRs, as well as for G protein and β-arrestin binding.
