Fig 5. The peripheral structures.
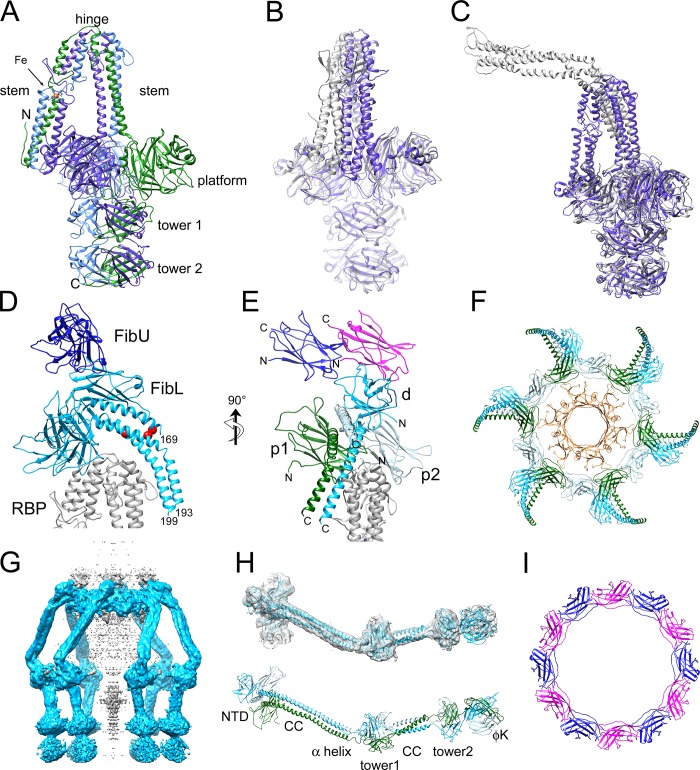
(A) Atomic model of the 80α RBP homotrimer. The three subunits are colored purple, blue and green. The stem, hinge, platform and two tower domains are indicated, as is an Fe atom (orange) coordinated by six His residues in the stem domain. (B) Superposition of 80α (purple) and ϕ11 (gray) RBPs, aligned by the tower and platform domains and rotated to emphasize the differing orientations of the stem domains. (C) Superposition of 80α RBP (purple) with the tail fiber (gp17) of P68 (gray), aligned by the tower domains. (D) Ribbon representation showing FibL (light blue), FibU (dark blue) and part of the RBP stem (gray). The positions of the K169 residues in the three FibL subunits are shown as red balls to indicate the relative shift of the three α-helices in the coiled coil. The C-terminal residue modeled in each FibL subunit is numbered. (E) Rotated view of D with the three FibL subunits colored green (p1), pale blue (p2) and blue (d). The two FibU subunits are in dark blue and magenta. RBP is gray. (F) Top view of baseplate showing the FibL ring octadecamer (colored as in E) and the MTP hexamer underneath (tan). (G) Isosurface (cutoff 3.0 σ) of a reconstruction made from signal-subtracted images excluding density corresponding to MTP, Dit, Tal, RBP and FibU. FibL density is blue. Additional density in the center (gray) probably corresponds to TMP. (H) Model for the complete FibL protein trimer built into the density from G. The lower panel is colored as in E, with structural elements labeled (CC, coiled coil; ϕK is the phage K gp68-like domain). (I) Top view of the dodecameric FibU ring, colored as in E.