Abstract
AIM:
The study aimed to isolate and identification secondary metabolite from pericarp Garcinia mangostana Linn.
METHODS:
The first step of this research was maceration of sample using alcohol 70% solvent. The separation and purification of compounds using Vacuum Liquid Chromatography (VLC), Radial Chromatography (RC). The purity of isolate was analyzed by thin layer chromatography (TLC) and melting point. Compounds identified using spectroscopi IR, NMR-1D (1H, 13C-NMR and DEPT) and 2-D NMR (HMQC and HMBC).
RESULTS:
The compound has melting point at 165-167°C. The result showed isolate was gartanin.
CONCLUSION:
The secondary metabolite found in pericarp Garcinia mangostana Linn. is gartanin.
Keywords: Pericarp mangosteen, Garcinia mangostana L, Gartanin
Introduction
Garcinia mangostana Linn. (mangosteen), is a functional plant because most of its parts can be used as medicine. Not only the fruit flesh which is consumed and believed to be beneficial for health, but according to research of mangosteen peel there are also a number of chemicals that are very beneficial for health [1]. G. mangostana Linn., Especially its peel has aroused interest for researchers to conduct intensive studies on the content of the compounds it contains. Mangosteen fruit peel is known to contain xanthon compounds which have the potential to be drug candidates. Xanthon is known to have antioxidants, anti-inflammatory activities [2], [3], antifungals [4], chemopreventions [2], [5], treatment of abdominal pain, diarrhea, dysentery, infection, pus, and chronic ulcers [6], anticancer [7], [8], antitumor [9], antimalarials [10], antiacne [11], antituberculosis [2], neuroprotective [12], antiproliferation [13], antimicrobial [14], cytoprotective [15], [16], anti-inflammatory [3]. Besides that, it also acts as an antioxidant [12], [15], [17], [16], [14].
Gartanin is the xanthon compound with the second most content after α-mangostin found in mangosteen, where the two compounds have the most role in biological activity (0.00082%). Gartanin has anti-cancer activities [18], antiviral influenza [19], antioxidants [20] and has strong activity against early stage lung cancer cells (NCIH187) [21]. Based on the above discussion, the purpose of this study is to isolate the content of pericarp mangosteen using vacuum liquid chromatography and radial chromatography.
Material and Methods
Specification of material
Plant material: one thousand g dry powder pericarp G. mangostana Linn. was collected at Somongari Village, Kaligesing District, Purworejo Regency, Central Java. Alcohol 70%, methanol (technical), acetone (technical), ethyl acetate (technical), n-hexane (technical), dichloromethane (technical), chloroform pa (E. Merck), silica gel 60 GF254 (E. Merck), silica gel 60 (0.2-0.5 mm) (E. Merck), silica gel 60 PF254 containing gypsum (E. Merck), distilled water, cerium sulfate (CeSO4), sulfuric acid (H2SO4). All solvents were distilled before being used, except chloroform. TLC was carried out using silica gel 60F254 (E. Merck) and visualized under UV light short and long (254 and 366 nm). Vacuum liquid chromatography was performed on silica gel 60 GF254 (E. Merck), the extract is impregnated with silica gel 60 (0.2-0.5 mm) (E. Merck), and radial chromatography was performed on silica gel 60 PF254 containing gypsum (E. Merck).
Instrumentation
A set of distillation apparatus (Duran-Germany), a set of vacuum liquid chromatography (VLC), radial chromatography (RC), vacuum rotary evaporator (Buchi), oven (Gallenkamp Civilab-Australia), analytical scales (Explorer Ohaus), UV lamps (Srahlen Germany). Melting points were measured on a Sybron Thermolyne Melting Point Apparatus MP-12615 and are uncorrected. FT-IR Spectra was on Perkin Elmer FT-IR Frontier. 1H and 13C-NMR spectra were recorded with an Agilent DD2 system (Agilent Technologies, Santa Clara, CA, USA) operating at 400 (1H) and 400 (13C) MHz using residual. Unless otherwise indicated, vacuum liquid chromatography, radial chromatography and TLC were carried out using Merck silica gel 60 GF254, silica gel 60 (finer than 0.2-0.5 mm) and precoated silica gel 60 PF254 containing gypsum plates, respectively. Spots on TLC were visualized under UV light and by spraying with cerium (IV) sulfate reagent followed by heating.
Procedure
Five thousand g G. mangostana Linn. pericarp powder macerated with 70% alcohol (5 L) then filtered. The collected filtrate is concentrated by using a vacuum rotary evaporator until is obtained a thick brown extract (250 g), then TLC eluent optimization was carried out in order to determine the mobile phase to be used during separation and purification. 250 g of pericarp mangosteen ethanol extract (GME) was separated by using vacuum liquid chromatography (VLC) 10 times (adsorbent silica column 300 g while the ratio of sample weight and silica impregnation was 1: 2 (25 g GME ethanol extract + 50 g) silica impregnation) and eluted with gradient polarity solvent steps starting from n-hexane, n-hexane-EtOAc, EtOAc and MeOH give five fractions (F1, F2, F3, F4 and F5). Based on the compound stain pattern, further separation of fraction 2 is carried out. Because of the large number (F2 50 g), the next step is to do the KVC 2 times, then the results of the KVC are combined so that 4 subfractions of F2 (F2.1 (100 mg); F2.2 (31.32 g); F2.3 (12.94 g) and F2.4 (457 mg) Subfraction F2.2 was purified by radial chromatography 6 times using plate 2 and the mobile phase used n-hexane: EtOAc (8: 2). Based on the same stain pattern then the results were combined again, then subfraction F2.3 too Radial chromatography was performed 4 times (same treatment F2.2). The fraction eluted from radial chromatography monitored by TLC and the fraction which gave a similar KLT pattern was combined so that 7 simple subfractions were obtained. Subfraction 2 from the combined results, purified by radial chromatography and obtained compound 1 as much as 45 mg (1,3,5,8-tetrahydroxy-2,4-bis (3-methylbut-2-en-1-yl) -9H- xanthen-9-one or Gartanin).
Identification of Isolates
The pure compounds obtained were measured and collected by using various spectrometry methods, namely FT-IR, 1-D NMR (1H, 13C and DEPT) and 2D NMR (HMBC and HMQC). The data obtained is translated by looking at the literature so that the structure can be known.
Results
Separation and purification in isolation of chemical compounds was carried out using chromatographic techniques. Chromatography is a way of physical separation with the elements to be separated distributed between two phases, the stationary phase and the mobile phase. The result of the separation can be seen in Figure 1.
Figure 1.

Chromatogram merging the fraction VLC result
Figure 1 shows that there are several fractions with compounds that have a very high Rf value which indicates that the fraction contains very nonpolar compounds. Separation prioritizes the factions that still have the most stains, the results of which are then combined with other factions with fewer stains. Separation by liquid vacuum column chromatography continued until a simpler subfraction was obtained, then purified using radial chromatography to obtain a single stain.
Purity Test and Melting Point
The amount of pure isolates obtained was 45 mg. Testing the purity of isolate 1 was done by TLC using three eluent systems, the stains of these compounds on the 3 types of eluents used can be seen in Figure 2.
Figure 2.

Chromatogram of three eluent system
The chromatogram in Figure 2 shows that isolate 1 has a single stain on the 3 three of eluent systems used. This shows that the compound has been pure and is supported by the results of the melting point test that has been carried out. The melting point of isolate 1 gave quite sharp results, namely at a temperature of 165-167°C.
Discussion
TLC profile of the pericarp G. mangostana Linn. under UV light (254 nm) showed only the presence of one major spot of ɑ-mangostin, one minor less polar spots and one minor more polar spot compared to that of ɑ-mangostin. Isolation work on this EtOAc soluble fraction of the pericarp mangosteen gave the one minor less polar compounds in a very small quantity compared to that of ɑ-mangostin. Attempt to isolate another more polar minor compound was unsuccessful because the amount was too small to isolate. Identification of the isolated compounds was done by spectroscopic method particularly NMR 1D (1H, 13C-NMR, and DEPT) and 2D (HMBC and HMQC).
Only the presence of two aromatic protons was observed (d, ppm, multiplicity, coupling constant); 7.22 (1H, d, J = 8.5 Hz) and 6.65 (1H, d, J = 9 Hz) which coupled to each other with coupling constant 7 Hz, indicating the presence of ortho-coupling of protons H6 and H7. The presence of two prenyl functions were also obvious by the signals of 4 methyl group (C-14; C-15; C-19 dan C-20) (d, ppm, multiplicity); 25.9, (3H, s), 18.1 (3H, s), 18.1 (3H, s), 25.8 (3H, s). There is no methoxyl signals were detected. Together with its 13C chemical shifts this compound was identified as known compound gartanin [22].
The pure compound 1 was obtained in the form of a yellow pale needle, dissolved in chloroform, and ethyl acetate but insoluble in n-hexane. IR (KBr) (cm-1): 3429, 3269, 3060, 2986, 2820, 1643, 1583, 1455, 1378, 1232, 1192, 1075, 986, 859. Through literature search, can be proven by comparing NMR data from compound (1) with gartanin from the library, as in Figure 3 and Table 1.
Figure 3.
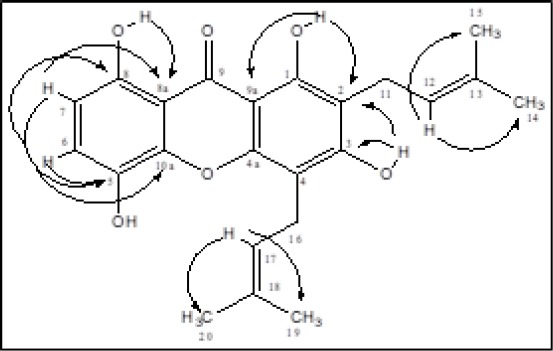
HMBC structure of compound (1)
Table 1.
1H dan 13C-NMR (400 MHz) spectral data of compound (1) and their references
| No C | 1H- NMR (ΣH, m, J in Hz) | 13C-NMR | DEPT 135 | HMBC | ||
|---|---|---|---|---|---|---|
| Compound (1) | Gartanin *) | (1) | *) | (1) | (1) | |
| 1 | 158.2 | 158.1 | Cq | |||
| 2 | 109.5 | 109.5 | Cq | |||
| 3 | 161.7 | 161.6 | Cq | |||
| 4 | 105.8 | 105.8 | Cq | |||
| 4a | 152.5 | 152.5 | Cq | |||
| 5 | 135.7 | 135.7 | Cq | |||
| 6 | 7.22 (1H, d, J = 8.5) | 7.22 (1H, d, J = 8.5) | 122.9 | 122.8 | CH | C8, C10a, C5 |
| 7 | 6.65 (1H, d, J = 9) | 6.63 (1H, d, J = 9) | 109.8 | 109.8 | CH | C5, C8 |
| 8 | 153.8 | 153.9 | Cq | |||
| 8a | 107.1 | 107 | Cq | |||
| 9 | 184.8 | 184.7 | Cq | |||
| 9a | 102.2 | 102.0 | Cq | |||
| 10a | 142.8 | 142.2 | Cq | |||
| 11 | 3.46 (2H, d, J = 7) | 3.46 (2H, d, J = 7) | 21.6 | 21.6 | CH2 | C12, C13, C1, C3 |
| 12 | 5.26 (1H, m, J = 7) | 5.23 (1H, m, J = 7) | 121.1 | 121.0 | CH | C15, C14 |
| 13 | 136.4 | 136.5 | Cq | |||
| 14 | 1.86 (3H, s) | 1.8 (3H, br s) | 26.0 | 25.9 | CH3 | C12, C13 |
| 15 | 1.79 (3H, s) | 1.86 (3H, br s) | 18.1 | 18.0 | CH3 | C13 |
| 16 | 3.52 (2H, d, J=6.5) | 3.51 (2H, d, J=6) | 21.7 | 22.1 | CH2 | C17, C18, C4a |
| 17 | 5.26 (1H, m, J = 7) | 5.23 (1H, m, J = 6) | 121.9 | 121.8 | CH | C19, C20 |
| 18 | 134.0 | 133.9 | Cq | |||
| 19 | 1.76 (3H, s) | 1.8 (3H, br s) | 18.1 | 18.0 | CH3 | C18 |
| 20 | 1.86 (3H, s) | 1.86 (3H, br s) | 25.8 | 25.9 | CH3 | |
| 1-OH | 12.35 | 12.34 | ||||
| 3-OH | 6.6 | 6.58 | ||||
| 5-OH | 5.02 | |||||
| 8-OH | 11.26 | 11.26 | ||||
Description: a) Compound 1; b) Anggia et al., 2015 [23].
In conclusion, the results of isolation and identification showed that in G. mangostana Linn. peel contained gartanin compounds.
Acknowledgment
We are grateful to DIKTI-Kemenristek “Postgraduate Team 2018” for supporting the preliminary study of Garcinia mangostana Linn. We thank B2P2TOOT for obtaining determination plant and standarisasion parameter specific and nonspecific. We thank Ratih Dewi Saputri, S.Si., M.Si from Airlangga University for obtaining NMR spectroscopic data.
Footnotes
Funding: This research was financially supported by the DIKTI-Kemenristek “Postgraduate Team 2018”, Indonesia
Competing Interests: The authors have declared that no competing interests exist
Authors’ Contributions
The writer and as the researcher of this paper is a postgraduate student of Faculty of Pharmacy in Setia Budi University, Indonesia.
References
- 1.Darmawansyih The Efficacy of Pericarp Mangosteen for Life. Journal Al Hikmah. 2014;15(1):1–9. [Google Scholar]
- 2.Chin Y, Jung H, Chai H, Keller W, Kinghorn A. Xanthones with quinine reductase-inducing activity from the fruits of Garcinia mangostana (Mangosteen) Phytochemistry. 2008;69(3):754–758. doi: 10.1016/j.phytochem.2007.09.023. https://doi.org/10.1016/j.phytochem.2007.09.023 PMid:17991497. [DOI] [PubMed] [Google Scholar]
- 3.Chomnawang MT, Surassmo S, Nukoolkarn VS, Gritsanapan W. Effect of Garcinia mangostana on inflammation caused by Propionibacterium acnes. Fitoterapia. 2007;78(6):401–405. doi: 10.1016/j.fitote.2007.02.019. https://doi.org/10.1016/j.fitote.2007.02.019 PMid:17644272. [DOI] [PubMed] [Google Scholar]
- 4.Gopalakrishnan B, Benumathi B, Suresh G. Evaluation of the antifungal activity of natural xanthones from Garcinia mangostana and their synthetic derivatives. J Nat Prod. 1997;60(5):519–524. doi: 10.1021/np970165u. https://doi.org/10.1021/np970165u PMid:9213587. [DOI] [PubMed] [Google Scholar]
- 5.Akao Y, Nakagawa Y, Iinuma M, dan Nozawa Y. AntiCancer Effects of Xanthones from Pericarps of Mangosteen. Int. J. Mol. Sci. 2007;9:355–370. doi: 10.3390/ijms9030355. https://doi.org/10.3390/ijms9030355 PMid:19325754 PMCid:PMC2635669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pedraza-Chevierri J, Cardenas-Rodriguez N, Orozco-Ibarra M, Perez-Rojas JM. Medicinal properties of mangosteen (Garcinia mangostana) Food Chem Toxicol. 2008;46:3227–3239. doi: 10.1016/j.fct.2008.07.024. https://doi.org/10.1016/j.fct.2008.07.024 PMid:18725264. [DOI] [PubMed] [Google Scholar]
- 7.Moongkarndi P, Kosem N, Kaslungka S, Luarantana O, Pongpan N, Neungton N. Antiploriferation, Antioxidant, and Induction Apoptosis by Garcinia mangostana on SKBR3 Human Breast Cancer Cell Line. Jurnal Ethnopharmacol. 2004;90:161–166. doi: 10.1016/j.jep.2003.09.048. https://doi.org/10.1016/j.jep.2003.09.048 PMid:14698525. [DOI] [PubMed] [Google Scholar]
- 8.Akao Y, Nakagawa Y, Iinuma M, dan Nozawa Y. AntiCancer Effects of Xanthones from Pericarps of Mangosteen. Int. J. Mol. Sci. 2004;9:355–370. doi: 10.3390/ijms9030355. https://doi.org/10.3390/ijms9030355 PMid:19325754 PMCid:PMC2635669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang Hui-Fang, Wen-Tsung Huang, Hui-Ju Chen, Ling-Ling Yang. Apoptotic Effects of γ-Mangostin from the Fruit Hull of Garcinia mangostana on Human Malignant Glioma Cells. Molecules. 2010;15:8953–8966. doi: 10.3390/molecules15128953. https://doi.org/10.3390/molecules15128953 PMid:21139533 PMCid:PMC6259202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahabusarakam W, Kuaha K, Wilairat P, dan Taylor WC. Prenylated Xanthone as Potential Antiplsamodial Subtance. Planta Medica. 2006;72:912–916. doi: 10.1055/s-2006-947190. https://doi.org/10.1055/s-2006-947190 PMid:16902859. [DOI] [PubMed] [Google Scholar]
- 11.Pothitirat Werayut, Mullika TC, Roongtawan S, Wandee G. Comparison of bioactive compounds content, free radical scavenging and anti-acne inducing bacteria activities of extracts from the mangosteen fruit rind at two stages of maturity. Fitoterapia. 2009;80:442–447. doi: 10.1016/j.fitote.2009.06.005. https://doi.org/10.1016/j.fitote.2009.06.005 PMid:19524646. [DOI] [PubMed] [Google Scholar]
- 12.Weecharangsan W, Opanasopit P, Sukma M, Ngawhirunpat T, Sotanaphun U, dan Siripong P. Antioxidative and Neuroprotective Activities of Extracts from the Fruit Hull of Mangosteen (Garcinia mangostana Linn.) Med Princ. Pract. 2006;15:281–287. doi: 10.1159/000092991. https://doi.org/10.1159/000092991 PMid:16763395. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto Kenji, Yukihiro Akao, Hong Yi, Kenji Ohguchi, Tetsuro Ito, Toshiyuki Tanaka, Emi Kobayashi, Munekazu Iinuma, Yoshinori Nozawa. Preferential Target is Mitochondria in α-Mangostin-Induced Apoptosis in Human Leukemia HL60 Cells. Bioorganic & Medicinal Chemistry. 2004;12:5799–5806. doi: 10.1016/j.bmc.2004.08.034. https://doi.org/10.1016/j.bmc.2004.08.034 PMid: 15498656. [DOI] [PubMed] [Google Scholar]
- 14.Palakawong C, Sophanodora P, Pisuchpen S, dan Phongpaichit Antioxidant and Antimicrobial Activities of Crude Extracts from Mangosteen (Garcinia mangostana L.). Parts and Some Essential Oils. International Food Research Journal. 2010;17:583–589. [Google Scholar]
- 15.Kosem N, Han YH, dan Moongkarndi P. Antioxidant and Cytoprotective Activities of Methanolic Extract from Garcinia mangostana Hulls. Science Asia. 2007;33:283–292. https://doi.org/10.2306/scienceasia1513-1874.2007.33.283. [Google Scholar]
- 16.Ngawhirunpat T, Opanasopi P, Sukma M, Sittisombut C, AtsushiKat dan Adachi I. Antioxidant, Free Radical-Scavenging Activity and Cytotoxicity of Different Solvent Extracts and Their Phenolic Constituents from The Fruit Hull of Mangosteen (Garcinia mangostana) Pharmaceutical Biology. 2010;48(1):55–62. doi: 10.3109/13880200903046138. https://doi.org/10.3109/13880200903046138 PMid:20645756. [DOI] [PubMed] [Google Scholar]
- 17.Zarena AS, dan Sankar KU. Study of Antioxidant Properties from Garcinia mangostana L. Pericarp Extract, Acta Sci. Pol. Technol. Aliment. 2009;8(1):23–34. [Google Scholar]
- 18.Liu Z, Antalek M, Nguyen L, Li X, Tian X, Le A, Zi X. The Effect of Gartanin, A Naturally Occurring Xanthone In Mangosteen Juice, on The Mtor Pathway, Autophagy, Apoptosis, And the Growth of Human Urinary Bladder Cancer Cell Lines. Nutr Cancer. 2013;65(1):68–77. doi: 10.1080/01635581.2013.785011. https://doi.org/10.1080/01635581.2013.785011 PMid:23682785 PMCid:PMC3671488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikram NK, Durrant JD, Muchtaridi M, Zalaludin AS, Purwitasari N, Mohamed N, Rahim AS, Lam CK, Normi YM, Rahman NA, Amaro RE, Wahab HA. A virtual screening approach for identifying plants with anti H5N1 neuraminidase activity. J Chem Inf Model. 2015;55(2):308–316. doi: 10.1021/ci500405g. https://doi.org/10.1021/ci500405g PMid:25555059 PMCid:PMC4340357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutierrez-Orozco F, Failla ML. Biological Activities and Bioavailability of Mangosteen Xanthones:A Critical Review of the Current Evidence. Nutrients. 2013;5(8):3163–3183. doi: 10.3390/nu5083163. https://doi.org/10.3390/nu5083163 PMid:23945675 PMCid:PMC3775248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suksamrarn Sunit, Orapin Komutiban, Piniti Ratananukul, Nitirat Chimnoi, Nattapat Lartpornmatulee, Apichart Suksamrarn. Cytotoxic Prenylated Xanthones from the Young Fruit of Garcinia mangostana. Chem. Pharm. Bull. 2006;54(3):301–305. doi: 10.1248/cpb.54.301. https://doi.org/10.1248/cpb.54.301 PMid:16508181. [DOI] [PubMed] [Google Scholar]
- 22.Ragasa CY, Crisostomo CJJ, Garcia KDC, Shen CC. Philippine Sci. 2010;47:63–75. [Google Scholar]
- 23.Anggia Vivi, Amri Bakhtiar, Dayar Arbain. The Isolation of Xanthones from Trunk Latex of Garcinia Mangostana Linn. And Their Antimicrobial Activities. Indones. J. Chem. 2015;15(2):187–193. https://doi.org/10.22146/ijc.21213. [Google Scholar]


