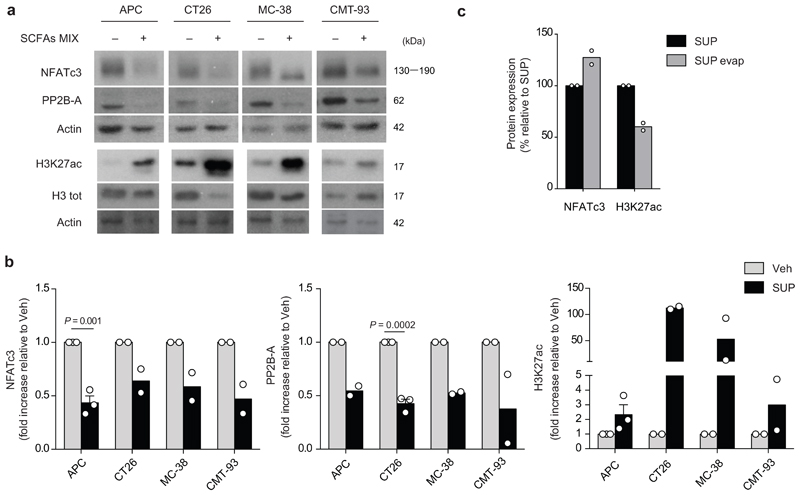
Extended Data Fig. 5. SCFAs and F. PB1 spent medium (SUP) increase histone H3 acetylation and reduce NFATc3-calcineurin pathway in vitro on mouse intestinal tumor cell lines.
a, Representative WB showing H3K27 acetylation, PP2B-A and NFATc3 in cell lines treated (+) or not (-) with a mix of SCFAs. Three independent experiments were performed with consistent results. b, Densitometric quantification of WB in Fig. 4D showing NFATc3 and PP2B-A (normalized to actin) and H3K27 acetylation (normalized to vinculin). Two or three independent experiments were performed with consistent results (n = 2 or n = 3 biologically independent experiments). Data are represented as means ± s.e.m. and P values were determined by two-tailed unpaired t-test. c, Densitometric quantification of WB in Fig. 4E showing NFATc3 (normalized to vinculin) and H3K27 acetylation (normalized to H3 tot). To calculate the protein expression induced by SUP evap as a percentage, the densitometric value of SUP was assumed to be 100%. Data from two independent WB (n = 2 biologically independent experiments).