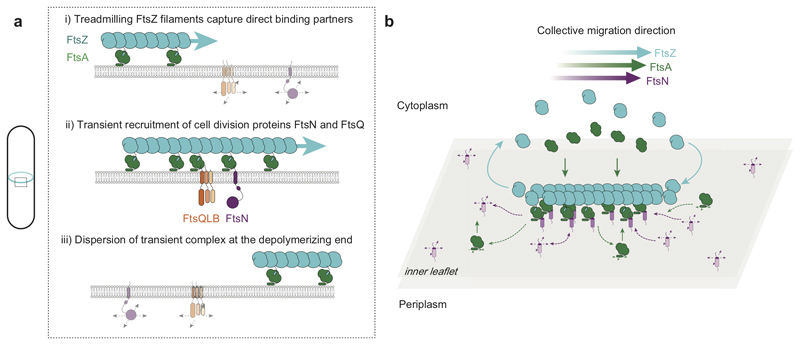
Figure 4. Schematic illustration of coupling between treadmilling FtsZ filaments and cell division proteins.
a. At early stages of cell division, treadmilling FtsZ filaments in the Z-ring act as a dynamic scaffold to recruit transmembrane proteins freely diffusing in the membrane. Both, FtsN and FtsQ directly bind to the FtsZ-FtsA co-filament to form a transient, stationary complex, which can then recruit other division proteins to the division site via interactions in the membrane or periplasm. Due to the local increase in concentration, weak interactions between proteins can be sufficient to initiate assembly of the division machinery. b. Illustration of proposed mechanism of co-migration. Freely diffusing FtsN in the membrane binds to the treadmilling FtsZ-FtsA filament on the membrane surface. While proteins reversibly bind along the whole length of the filaments, there is a net accumulation of proteins at the growing end. In contrast, proteins rapidly disperse at the depolymerizing end. This dynamic coupling to the treadmilling filaments permits a collective co-migration on the ensemble level while individual proteins show uncorrelated behavior.