Figure 1.
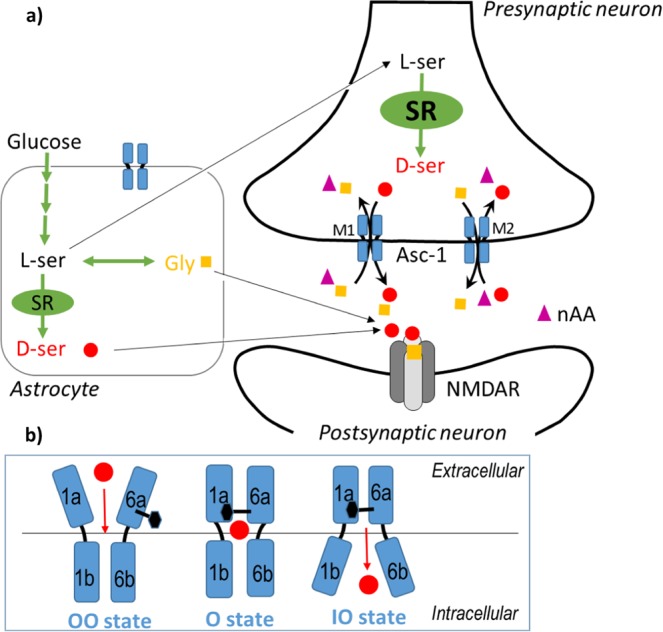
Central role of Asc-1 at the tripartite glutamatergic synapse. (a) It has been reported that the Asc-1 transporter (blue) plays an important role in regulating NMDAR-dependent synaptic activity by mediating release of d-serine (red) in exchange to other neutral amino acids (violet) including glycine (yellow). Activation of the GluN1-GluN2 containing NMDA receptors (NMDAR, grey) in addition to glutamate requires the binding of a co-agonist d-serine and/or glycine. Interestingly, the binding of glycine overlaps with the d-serine binding at synaptic NMDARs. Also, d-serine is formed from l-serine by Serine Racemase (SR, green). Transport of d-serine including the efflux of the substrate (indicated by M1 mechanism) along with the uptake of the substrate (indicated by M2 mechanism) across the membrane involves roughly three states: outward-open (OO), occluded (O) and inward-open (IO) (Fig. 1b) with additional intermediate states such as outward-open occluded (OOO) and inward-open occluded (IOO) (not shown).
