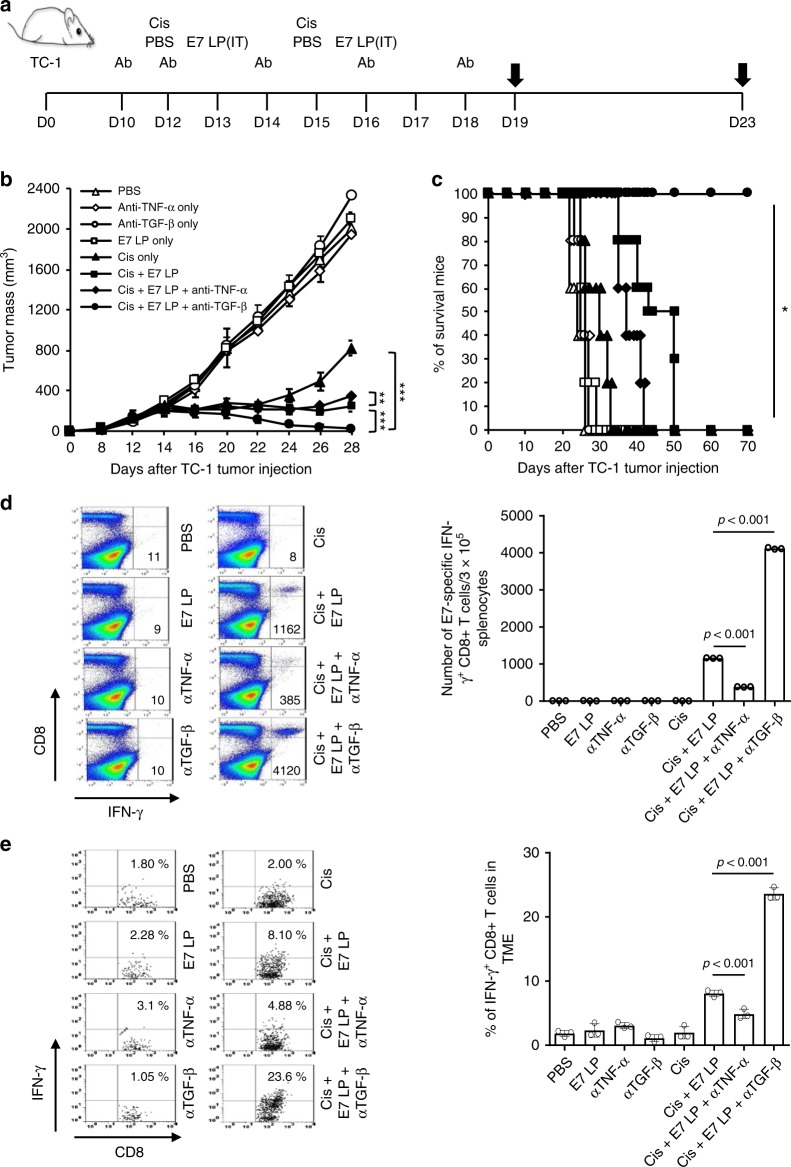
Fig. 3. Antitumor effect of anti-TGF-β or anti-TNF-α antibody treatment.
C57BL/6 mice were injected with 2 × 105 TC-1 cells/mice subcutaneously on day 0. Mice were then treated intraperitoneally with 200jig/mice TGF-3 or TNF-α neutralizing antibody on day 10, 12, 14, 16, and 18, intraperitoneally with 5 mg/kg Cisplatin on days 12 and 15, and/or intratumorally with 20jig/mice of E7 long peptide on days 13 and 16. The treatment groups are as follows: opened triangle - PBS only; opened sphere - anti-TNF-α only - opened circle, TGF-3 only - opened square, E7 long peptide only - closed triangle, cisplatin only - closed square, cisplatin and E7 long peptide - closed sphere, cisplatin, E7 long peptide, and anti-TNF-α; closed circle—cisplatin, E7 long peptide, and anti-TGF-f3. a Schematic diagram. b Line graph depicts TC-1 tumor growth in different treatment groups over time (n = 10). P-values were determined by one-way ANOVA and Turkey’s test. (c) Kaplan–Meier survival analysis of TC-1 tumor-bearing mice in different treatment groups (n = 10), and the overall P-value was calculated by the log-rank test. d, e On days 19 and 23, tumor tissues and spleens of TC-1 tumor-bearing mice in different treatment groups were harvested and analyzed for CD8+IFN-γ+ T cells by flow cytometry analysis, respectively. d Representative flow cytometry analysis and bar graph depicting the abundance of CD8+IFN-γ+ T cells in splenocytes of TC-1 tumor-bearing mice in different treatment groups (n = 3). (e) Representative flow cytometry analysis and bar graph depicting the abundance of CD8+IFN-γ+tumor-infiltrating T cells in TC-1 tumor-bearing mice in different treatment groups (n = 3). The error bars indicate mean ± SD. For d, e, P-values were analyzed by Student’s t test. The results are representative of one of three independent experiments. Source data are provided as a Source Data file.