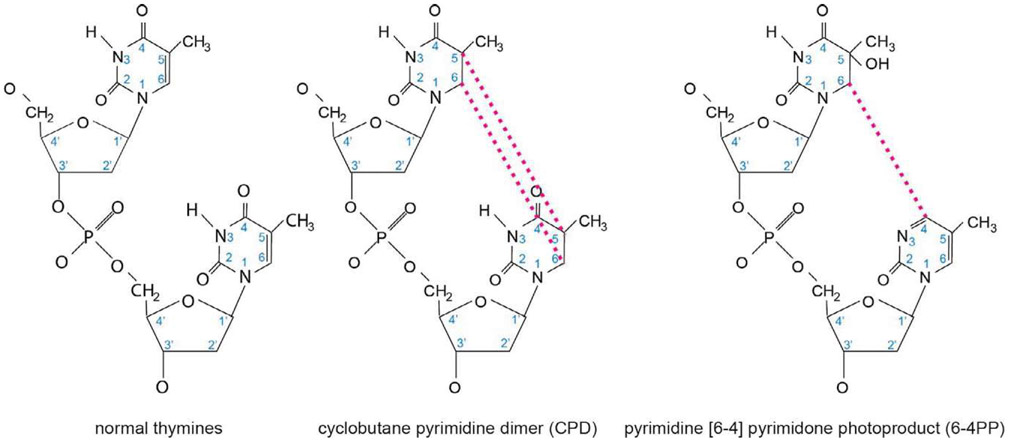
Fig. 8.
Chemical structures of UV photoproducts. When UV-light strikes DNA at dipyrimidine nucleotides, such as the thymine dimer shown on the left, there are two common DNA lesions formed. First, the 5′ and 6′ positions of the pyrimidine ring structures can form a cyclobutane ring between the adjacent thymines, generating a DNA lesion referred to as a cyclobutane pyrimidine dimer (CPD) as shown in the middle panel. This lesion is the less helically distorting, and therefore more poorly repaired, of the two adducts shown, and is the most commonly generated adduct when DNA is exposed to UV-light. The second most commonly generated DNA adduct is the pyrimidine [6–4] pyrimidone photoproduct (6–4PP), a lesion formed when the 6′ carbon of one thymine covalently binds the 4′ carbon of an adjacent thymine, as shown in the panel on the right. The 6–4PP is considerably more helically distorting than the CPD lesion, and is therefore more readily recognized by NER, more rapidly repaired, and consequently less mutagenic than a CPD lesion.