Fig. 2. Structural characterization of multidomain peptides.
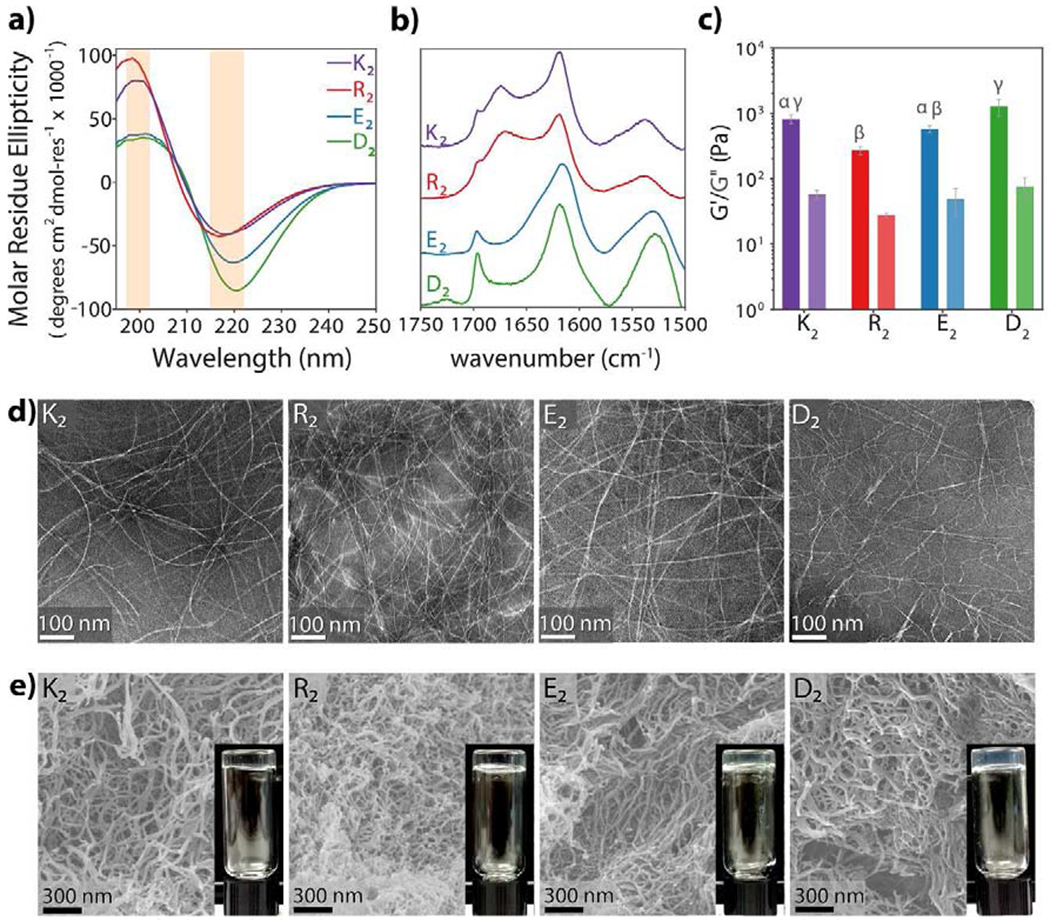
a) Circular dichroism spectroscopy of 1% by weight peptide hydrogels shows characteristic β-sheet spectra with minima near 218 nm and maxima near 196 nm. b) Normalized ATR-FTIR spectra of dried peptide films confirm the antiparallel β-sheet secondary structure of all MDPs by the presence of an amide Ia peak at 1620–1630 cm−1 and an amide Ib peak near 1695 cm−1 c) Viscoelastic properties of MDP hydrogels analyzed by oscillatory rheology. MDPs form soft hydrogels. Error bars represent standard deviation. Different greek letters represent a significant difference, p-value < 0.05. d) MDPs self-assemble into long flexible nanofibers as observed in negative-stained TEM. e) SEM images of peptide hydrogels. Peptide nanofibers are stabilized and physically crosslinked by the presence of ions, forming a highly entangled nanofibrous network and a self-supportive hydrogel.
