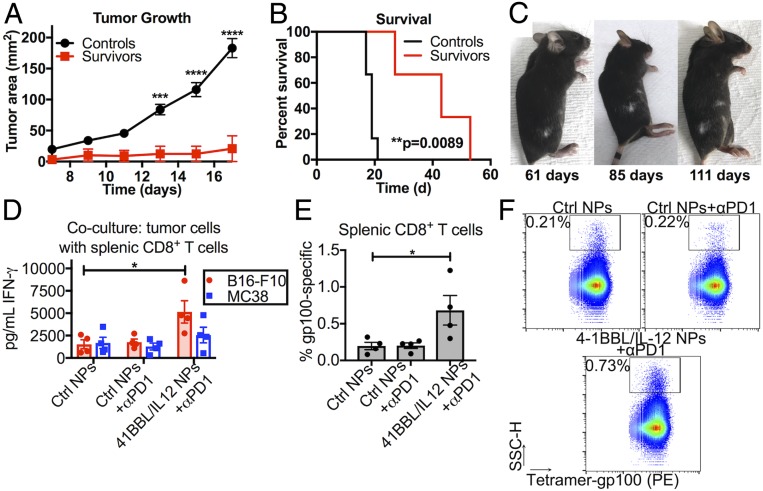
Fig. 6.
Local tAPC reprogramming leads to a durable and systemic antitumor immune response. (A) Survivors rechallenged with new s.c. B16-F10 tumors on the opposite flank resisted tumor formation compared to untreated control mice and (B) survived significantly longer after rechallenge. **P < 0.01; ***P < 0.001; ****P < 0.0001. (C) One of the long-term survivors developed a vitiligo-like patch of depigmented fur at the site of the eliminated tumor, which began to spread to other patches of fur, indicating a cytotoxic immune response at more distant locations. Statistically significant differences in the growth rate were measured by two-way repeated-measures t tests with Holm–Sidak tests to correct for multiple comparisons. Differences in survival were calculated by the Mantel–Cox log-rank test. (D) CD8+ T cells isolated from tAPC-treated tumor-bearing mice were activated more effectively after in vitro stimulation with B16-F10 cells than CD8+ T cells from tumor-bearing mice administered control nanoparticles or checkpoint inhibition alone. (E and F) The splenic CD8+ T cell population was more specific for gp100, a common melanoma antigen. PE, phycoerythrin. The graphs show mean ± SE. Significance was calculated by one-way ANOVA with Dunnett posttests against the “Ctrl NPs” group. *P < 0.05.