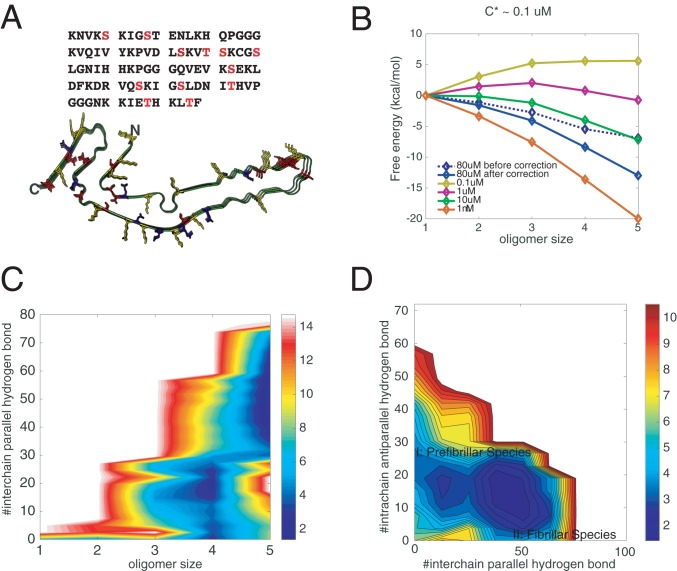
Fig. 3.
Effects of phosphorylations on the aggregation of R1/3/4. (A) The amino acid sequence of R1/3/4 in tau. All of the potential phosphorylated serines/threonines are shown in red. The solved fibrillar structure of R1/3/4 is also illustrated, with positively charged residues colored in yellow, negatively charged residues in blue, and potentially phosphorylated sites in red. Three of the 12 residues are randomly phosphorylated during recalculating the free-energy surfaces using thermodynamic perturbation theory (SI Appendix, Fig. S2). (B) The perturbation-based grand canonical free-energy profiles for different oligomer states corrected for the monomer concentration changes in the fixed number simulation. (C) Perturbation-based grand canonical free-energy surface at the concentration of 80 M at 300 K is plotted using the number of intermolecular parallel hydrogen bonds and the oligomer size as the two dimensions. (D) The recalculated grand canonical free-energy surface is also plotted using the number of intermolecular parallel hydrogen bonds and the number of intramolecular antiparallel hydrogen bonds as the two dimensions to be compared with the landscape for the unphosphorylated assembly.