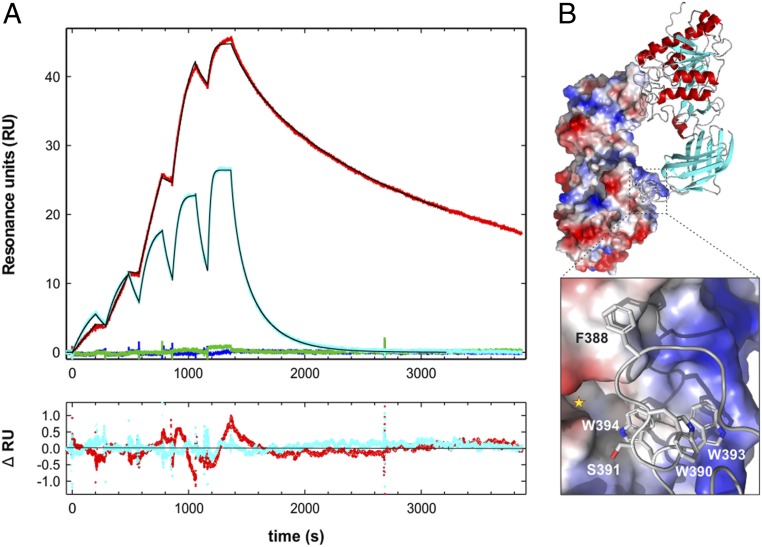
Fig. 1.
Epitope for mAb 5D2 on LPL defined by SPR. (A) Single-cycle SPR sensorgrams for the interaction between immobilized mAb 5D2 and various synthetic peptides corresponding to amino acid residues 383 to 396 of human LPL (KSDSYFSWSDWWSS). Red curve, the wild-type (wt) peptide sequence (1 to 16 nM); blue curve, Trp390→Ala (8 to 128 nM); cyan curve, Trp393→Ala (2 to 32 nM); green curve, Trp394→Ala (8 to 128 nM). Thin black lines represent fits to the data recorded for the wt and Trp393→Ala peptides. Residuals of these fits are shown at the bottom. (B) The structure of an LPL homodimer depicting the reciprocal interactions of two partner monomers, as revealed by X-ray crystallography (31). (Top) One LPL protomer in an electrostatic potential surface representation and the other in a cartoon representation. The hatched square highlights the location of one of the reciprocal LPL homodimer contacts. (Bottom) The corresponding close-up, depicting the involvement of residues 383 to 396 of human LPL in one of two low-energy models. The yellow asterisk marks the entrance to the catalytic site of the LPL protomer.