PLANT BIOLOGY Correction for “BBX4, a phyB-interacting and modulated regulator, directly interacts with PIF3 to fine tune red light-mediated photomorphogenesis,” by Yueqin Heng, Yan Jiang, Xianhai Zhao, Hua Zhou, Xuncheng Wang, Xing Wang Deng, and Dongqing Xu, which was first published November 27, 2019; 10.1073/pnas.1915149116 (Proc. Natl. Acad. Sci. U.S.A. 116, 26049–26056).
The authors wish to note the following: “We were made aware of mistakes in the labeling of the bottom two panels on the right side of Fig. 1E and Fig. 3C, and Fig. S4B in the SI Appendix. In these three figures, the yeast SD plate was labeled incorrectly as “-Trp/-Leu/-Trp/-Ade,” whereas the correct label should be “-Trp/-Leu/-His/-Ade.” For Fig. S4B, the image of yeast two-hybrid assays was presented incorrectly due to a typographical error introduced during revision. The legends for Fig. 1E, Fig. 3C, and Fig. S4B remain unchanged. This correction does not otherwise affect the results nor alter any of the original conclusions. We apologize for any inconvenience to the readers. All authors have approved this correction.” The corrected Fig. 1 and Fig. 3 appear below with their respective legends. The SI Appendix has been updated online and includes the corrected Fig. S4.
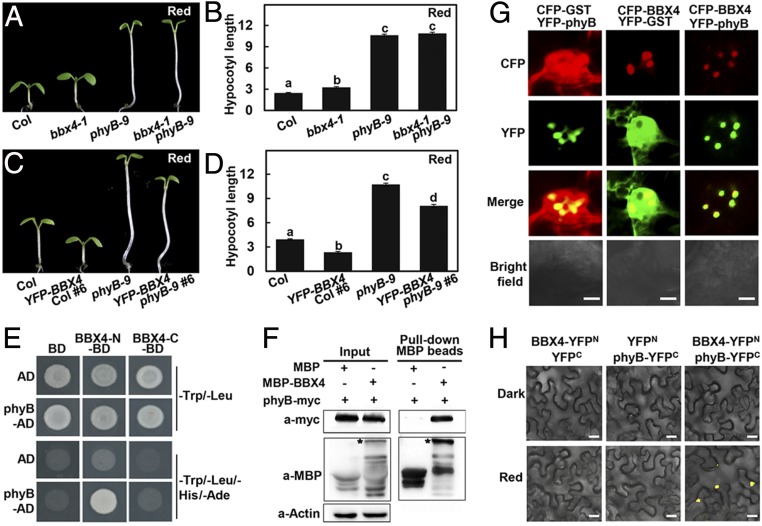
Fig. 1.
phyB genetically and physically interacts with BBX4. (A and B) Hypocotyl phenotype (A) and length (B) of 4-d-old Col, bbx4-1, phyB-9, and bbx4-1 phyB-9 seedlings grown in R (115.8 μmol/m2/s) light. The unit of hypocotyl length is millimeters. The experiments were performed 3 times with similar results. The graphs depict one of these experiments. Error bars represent SE (n ≥ 20). Letters above the bars indicate significant differences (P < 0.05), as determined by 1-way ANOVA with Tukey’s post hoc analysis. (C and D) Hypocotyl phenotype (C) and length (D) of 4-d-old Col, YFP-BBX4 #6, phyB-9, and YFP-BBX4 phyB-9 #6 seedlings grown in R light (115.8 μmol/m2/s). The unit of hypocotyl length is millimeters. The experiments were performed 3 times, with similar results. The graphs depict one of these experiments. Error bars represent SE (n ≥ 20). Letters above the bars indicate significant differences (P < 0.05), as determined by 1-way ANOVA with Tukey’s post hoc analysis. (E) Yeast two-hybrid interactions between the BBX4 and phyB. (F) Semi-in vivo pull-down assay of BBX4 with phyB. Total plant protein was extracted from 4-d-old phyB-myc transgenic seedlings grown in R light (115.8 μmol/m2/s). Equal amounts of MBP and MBP-BBX4 proteins were added to total plant protein extracts. The asterisk indicates MBP-BBX4. Actin served as a negative control. (G) BBX4 and phyB colocalize to the nuclear bodies in tobacco cells. CFP-BBX4 and YFP-phyB were transiently coexpressed in tobacco leaves. CFP-GST and YFP-GST served as negative controls. (Scale bars: 5 µm.) (H) BiFC assay showing the interaction of BBX4 with phyB in R light. BBX4 and phyB were fused to the N- and C-terminal fragments of YFP (YFPN and YFPC, respectively). Unfused YFPN and YFPC fragments served as negative controls. (Scale bars: 20 μm.)
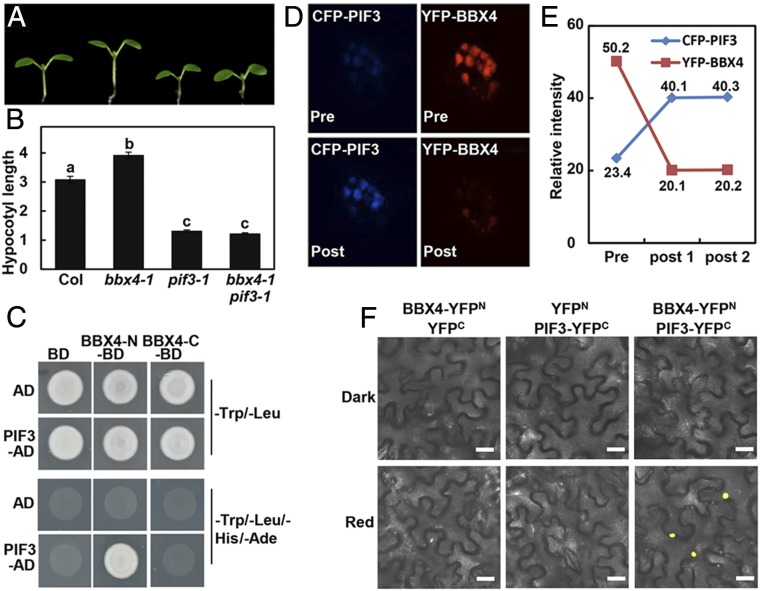
Fig. 3.
BBX4 genetically and physically interacts with PIF3. (A and B) Hypocotyl phenotype (A) and length (B) of 4-d-old Col, bbx4-1, pif3-1 and bbx4-1 pif3-1 seedlings grown in R light (115.8 μmol/m2/s). The unit of hypocotyl length is millimeters. The experiments were performed 3 times, with similar results. The graphs depict one of these experiments. Error bars represent SE (n ≥ 20). Letters above the bars indicate significant differences (P < 0.05), as determined by 1-way ANOVA with Tukey’s post hoc analysis. (C) Yeast two-hybrid interactions between the BBX4 and PIF3. (D) FRET between CFP-PIF3 and YFP-BBX4 analyzed by acceptor bleaching in nuclei. (Top) Representative prebleach nuclei coexpressing YFP-BBX4 and CFP-PIF3 excited with a 514-nm or 405-nm laser, resulting in emission from YFP or CFP, respectively. (Bottom) The same nuclei after bleaching excited with a 514-nm or 405-nm laser. (E) The relative intensities of both YFP and CFP inside the nuclei were measured once before and twice after the bleaching, as indicated in D. (F) BiFC assay showing the interaction of BBX4 with PIF3 in red light. BBX4 and PIF3 were fused to the N- and C-terminal fragments of YFP (YFPN and YFPC, respectively). Unfused YFPN and YFPC fragments served as negative controls. (Scale bars: 40 μm.)