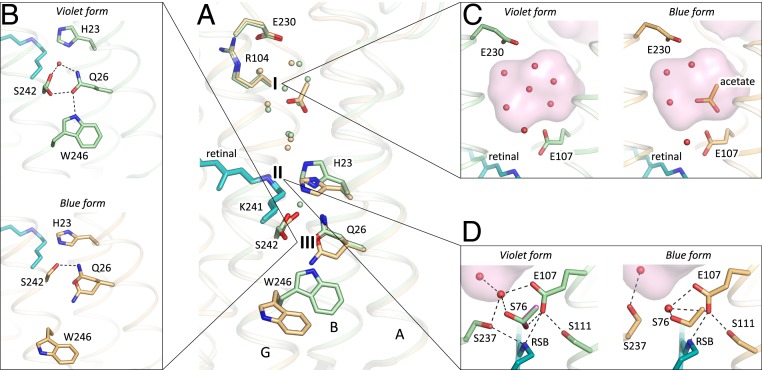
Fig. 5.
Comparison of the violet (shown in green) and blue (shown in orange) forms of 48C12. (A) Alignment of the two models. The three most notable differences between two structures are 1) the cavity at the cytoplasmic side, 2) rearrangements of the residues near the RSB, and 3) loss of the water molecule between His23 and Ser242 in the blue form and rearrangements of the Gln26 and Trp246 side chains. Water molecules are shown with the spheres and colored green and orange, corresponding to the violet and blue forms of 48C12, respectively. (B) Detailed view of the Ser242-Gln26-Trp246 cluster and His23 in the violet and blue forms. (C) Detailed view of the cavity (active site) at the cytoplasmic side in the violet and blue forms. (D) Detailed view of the RSB and surrounding residues in the violet and blue forms. In violet form, Ser76 assumes two alternative conformations (second is colored magenta for clarity). Cavities are colored pink.