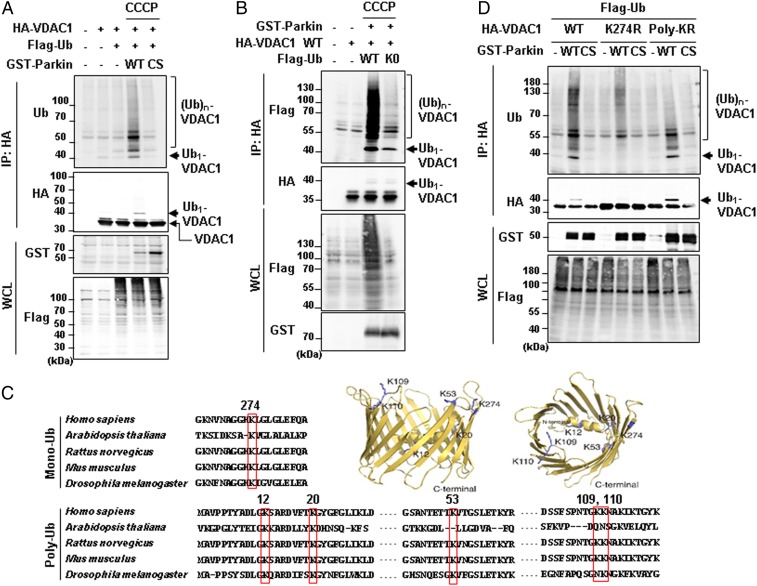
Fig. 1.
Mono- and polyubiquitination of VDAC1 are dependent on Parkin and PINK1 activity. (A, B, and D) Ubiquitination assays for VDAC1 by Parkin. HEK293T cells were transfected with indicated constructs and treated with or without 20 µM CCCP for 4 h. Whole-cell lysates (WCL) were immunoprecipitated (IP) and analyzed for IB analyses with indicated antibodies. The monoubiquitination band was marked on the right side and the polyubiquitination bands were marked by a bracket. Parkin WT and CS (C431S) mutants were expressed and compared in A and D. Flag-tagged ubiquitin WT and K0 (K6, 11, 27, 29, 33, 48, and 63R mutant) were expressed in B. VDAC1 WT and two ubiquitination mutants (VDAC1 K274R and Poly-KR) were expressed and also examined for Parkin-dependent ubiquitination assays in D. (C) Aligned VDAC1 protein sequences of human (VDAC1, P21796.2), Arabidopsis (VDAC1, NP_186777.1), rat (VDAC1, NP_112643.1), mouse (VDAC1 isoform 2, NP_035824.1), and Drosophila (Porin isoform E, NP_001260365.1) with indication of the conserved lysine residues in red boxes. Three-dimensional human VDAC1 protein structures, Protein Data Bank ID code 5JDP (P21796), were additionally used to predict the location of mono- and polyubiquitination sites.