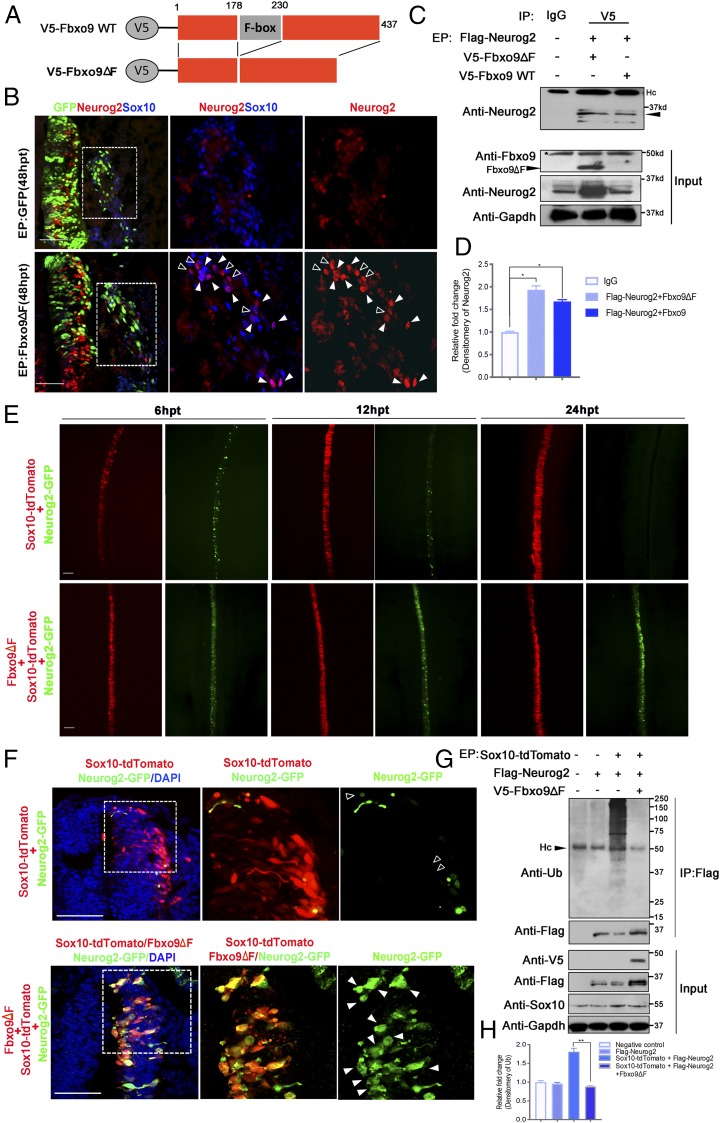
Fig. 7.
Mutant form of Fbxo9 stabilizes Neurog2 protein. (A) Structure of wild-type (WT) Fbxo9 and its mutant form without F-box domain. (B) Immunofluorescence for Neurog2 and Sox10 on cross-sections of embryos electroporated with GFP control (n = 7) or Fbxo9ΔF (n = 8) at 48 hpt. The magnified areas are marked with dashed boxes. Closed and open arrowheads indicate Sox10+Neurog2+ cells and cells expressing Neurog2 alone, respectively. (C) Well-transfected chicken embryos with the indicated constructs (n = 10 per treatment) were subjected to IP with anti-V5 and blotted with anti-Neurog2. A total of 20% of the total input was blotted with anti-Fbxo9 and anti-Neurog2 to detect both endogenous and ectopic levels of Fbxo9 and Neurog2 expression, respectively. Gapdh served as a loading control. Black arrowhead indicates endogenous and ectopic Neurog2 proteins pulled down by Fbxo9. Asterisk indicates endogenous Fbxo9 expression. (D) Densitometric quantification of the levels of immunoprecipitated endogenous and ectopic Neurog2 in each treatment relative to the control. (E) Dorsal view of chicken embryos transfected with Sox10-tdTomato+Neurog2-GFP (n = 7) and Sox10-tdTomato+Fbxo9ΔF+Neurog2-GFP (n = 8) at 6, 12, and 24 hpt. (F) Cross-sections of embryos electroporated with the indicated constructs at 24 hpt. The magnified areas are marked with dashed boxes. Closed and open arrowheads indicate persistence and reduced expression of Neurog2-GFP, respectively. (G) Well-transfected chicken embryos with the indicated constructs (n = 10 per treatment) were subjected to IP with anti-Flag and blotted with anti-Ub or anti-Flag. A total of 20% of the total input was blotted with anti-V5, anti-Flag, and anti-Sox10. Gapdh served as a loading control. (H) Densitometric quantification of the levels of Flag-Neurog2 conjugated to Ub in each treatment relative to the control. Hc, heavy chain. Error bars ± SEM (*P < 0.05, **P < 0.01). (Scale bars: embryos, 20 μm; sections, 50 μm.)