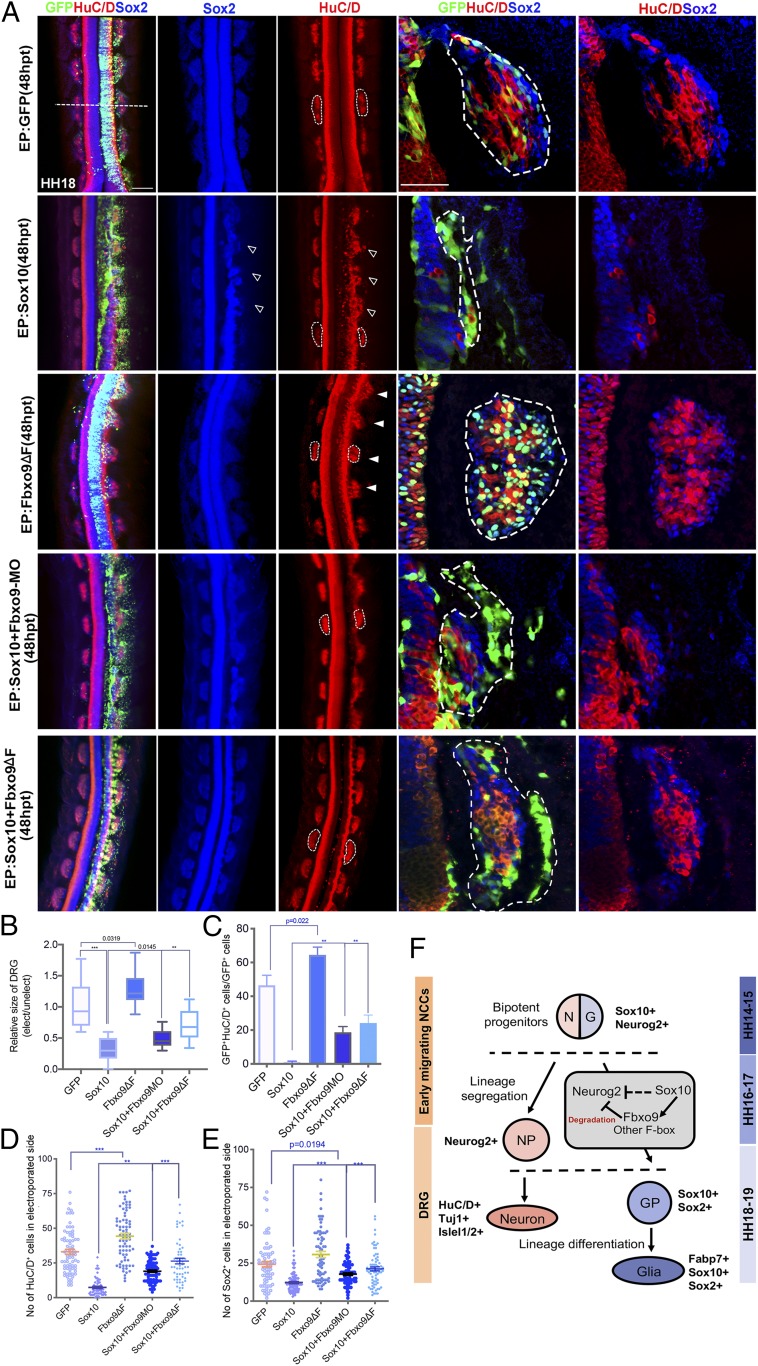
Fig. 8.
Fbxo9 and possibly other F-box members mediate the role of Sox10 to regulate neuron–glial cell fate choice in the DRG. (A) Immunofluorescence for HuC/D and Sox2 of chicken embryos transfected with GFP control (n = 7), Sox10 (n = 8), and Fbxo9ΔF (n = 7). Sox10+Fbxo9ΔF (n = 8) and Sox10+Fbxo9 MO (n = 8) at 48 hpt. Dotted lines indicate the plane of sectioning and outline the border of the DRG. Open arrowheads indicate DRG dysplasia. Solid white arrowheads indicate enlargement of DRG. (B) Graph showing fold differences in the size of the DRG between electroporated and unelectroporated sides of embryos treated with the indicated constructs. (C) Quantification of the number of GFP+HuC/D+ cells in embryos transfected with the indicated constructs. Quantification of the number of (D) HuC/D+ and (E) Sox2+ cells in the electroporated side of embryos treated with the indicated constructs. (F) Model of the role of Fbxo9 in regulating neuronal–glial cell fate choice in the DRG. Early-migrating bipotent NC progenitors transiently coexpressing Sox10 and Neurog2 undergo lineage segregation processes, in which Sox10 destabilizes Neurog2 (dotted line) through induced expression of Fbxo9 and other F-box factors, resulting in the acquisition of glial progenitor (GP) fate and subsequent differentiation into satellite glial cells, whereas Neurog2+ neuronal progenitors (NP) evade Sox10-mediated degradation to differentiate into sensory neurons within the core of the DRG. Error bars ± SEM (**P < 0.01, ***P < 0.001). (Scale bars: embryos, 10 μm; sections, 50 μm.)