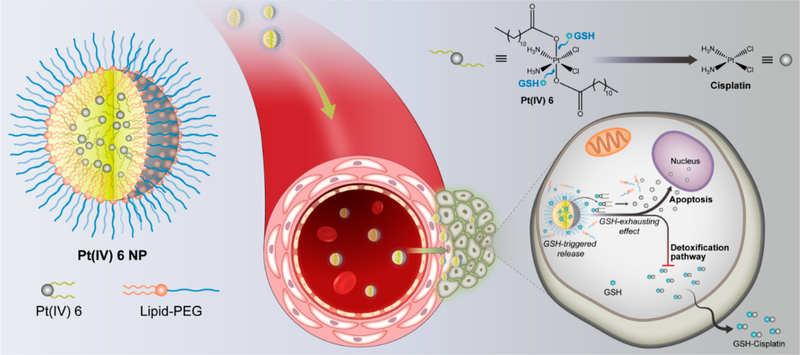
Figure 1.
Schematic illustration of self-assembled Pt(IV) NPs for specific delivery of Pt drugs and effective suppression of cisplatin-resistant tumors. Redox-responsive P6 NPs were self-assembled with superhydrophobic Pt(IV) 6 and coated with amphiphilic lipid-PEG via nanoprecipitation. Benefiting from the extended blood circulation and selective tumor accumulation, P6 NPs could be endocytosed into tumor cells through macropinocytosis and then disintegrated by consumption of cytoplasmic thiol-containing species, especially GSH. The redox-triggered process contributed to the release of Pt(II) ions and their reduced probability of deactivation, which went on to rapidly diffuse into nuclei and covalently bind large amounts of DNA, ultimately resulting in the mitochondria-controlled apoptosis of cisplatin-resistant tumors.