Abstract
Backgrounds and Aims
It is well known that angiogenesis contributes to the progression of chronic obstructive pulmonary disease (COPD) by initiating the remodeling of bronchial vasculature. However, the specific molecular mechanisms are incompletely understood. This research aimed to explore whether endostatin, a member of endogenous antiangiogenic proteins, is a biomarker in COPD and plays a role in the angiogenesis of COPD.
Methods
100 stable COPD patients, 130 patients with acute exacerbation (AECOPD) and 68 healthy volunteers were recruited in this research. Lung function test was conducted in the healthy people and stable COPD patients. Serum endostatin, C-reactive protein (CRP) and vascular endothelial growth factor (VEGF) of all the subjects were measured by Human Magnetic Luminex Screening Assay.
Results
Serum endostatin level was significantly higher in stable COPD compared with healthy control and even more in AECOPD patients (P<0.001). Besides, stable COPD patients with frequent exacerbation (≥2 exacerbations per year) in the last 1 year had a higher concentration of endostatin in the circulation compared to the patients with less exacerbation (P=0.037). Furthermore, circulatory endostatin was negatively associated with forced expiratory volume in 1 s % predicted (FEV1%pre), an index of lung function in the stable COPD group (P=0.009). Finally, endostatin was positively correlated to serum CRP in COPD group (including stable and AECOPD) (P=0.005) and all the subjects (P<0.001), but only associated with VEGF in the total participants (P=0.002), not in the COPD group.
Conclusion
These results suggested that endostatin is a biomarker for COPD and associated with lower lung function, exacerbation, and systemic inflammation. Endostatin potentially contributes to the pathogenesis of COPD.
Keywords: chronic obstructive pulmonary disease, endostatin, lung function, angiogenesis, inflammation
Introduction
Chronic obstructive pulmonary disease (COPD) is a common airway disease that is distinguished by constant respiratory symptoms, limitation of airflow, and a series of complications and comorbidities.1 It is one of the main causes of morbidity and mortality in the world, which has risen from the eighth to the fifth in terms of burden of disease from 1990 to 2013 and has been the fourth leading reason for global death in 2013.2 Its diagnosis depends on the fact that forced expiratory volume in 1 s to forced vital capacity ratio (FEV1/FVC) after bronchodilator is less than 70% with the particular symptoms and triggering risks.1 Apart from the local inflammation in the lung, COPD is also an endothelial and systemic inflammatory disease, which is linked to more severe symptoms, more frequent exacerbation, worse lung function or more comorbidities.3,4 Numerous factors have been reported to contribute to the process from local inflammation to systemic inflammation, which causes the self-continuous property of COPD without ongoing stimuli.3,5,6 Even though arguments about how they work in COPD still exist, research about these biomarkers did provide many novel ideas about the pathogenesis of COPD and might help to monitor and manage the progression of COPD.
Endostatin is one of the endogenous antiangiogenic proteins activated by proteolytic cleavage.7 It prevents endothelial cell proliferation, migration/invasion and tube formation.8 Some studies revealed that endostatin downregulated endothelial signaling pathways related to proangiogenic activity as well as upregulated the antiangiogenic genes.9 However, other studies suggested that endostatin had both anti- and pro-angiogenic functions depending on dosages and endothelial cell types.10 Despite its disparate mechanisms of actions and functions, it was reported to be elevated in certain cancers such as lung cancer and in some chronic inflammatory diseases including chronic kidney disease,11 systemic sclerosis,12 and diabetic retinopathy.13 Similarly, increased serum endostatin was related to adverse function, decreased activity tolerance and invasive hemodynamics indexes in pulmonary arterial hypertension (PAH).14 These suggest that endostatin may be also a potential biomarker for airway inflammatory diseases. Furthermore, Kanazawa et al reported an imbalance between vascular endothelial growth factor (VEGF) and endostatin levels in induced sputum from emphysema patients.15 Nevertheless, no research on circulatory endostatin of COPD has been conducted. Therefore, we designed this initial clinical study to explore the alteration of circulating endostatin in COPD and the association between endostatin with lung function and systemic inflammation of COPD.
Materials and Methods
Subjects
100 stable COPD patients from the outpatient department of West China Hospital, 130 patients with acute exacerbation (AECOPD) from the inpatient department of Third People’s Hospital in Chengdu and 68 healthy volunteers from the physical examination center of West China Hospital were recruited from June 2016 to March 2017. Stable COPD patients and healthy volunteers experienced standard pulmonary function test using the standardized method according to American Thoracic Society guidelines. AECOPD patients have been diagnosed as COPD before according to their lung function, but we did not test the pulmonary function when we recruited them because it could not reflect their baseline lung function when the patients had an exacerbation. COPD was diagnosed based on Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria: (a) FEV1/FVC < 70% and (b) the increase of forced vital capacity (FVC) or FEV1 < 12% or 200 mL after inspiring bronchodilator β-agonist (200 mg salbutamol). The basic health information of all the participants was gathered by performing questionnaires and physical examinations. Blood routine examination was performed for all the subjects. Patients who had pneumonia, tuberculosis, pulmonary fibrosis, malignant tumors, and rheumatology diseases within the last 12 weeks before recruitment were excluded. The study protocols complied with the principles of the Declaration of Helsinki and were approved by the Institutional Review Board for Human Studies of West China Hospital of Sichuan University, China (the approved number is 2015 (59)). Written informed consents were obtained from all the subjects.
Blood Collection and Assays
The fasting vein blood was taken from the cubital vein of all subjects and centrifuged at 3000 rpm for 10 mins at 4°C straightaway. Then, the serum was aliquoted and stored at −80°C until analysis. Levels of endostatin, C-reactive protein (CRP) and VEGF were detected using Human Magnetic Luminex Screening Assay (LXSAHM; R&D Systems, Inc. Minneapolis, MN, USA) on Bio-Plex 200 detection platform (Bio-Rad, California, USA) according to the manufacturer’s instructions. The lower limits of detection were 27.3 pg/mL for endostatin, 116pg/mL for CRP and 2.1pg/mL for VEGF. Operators performing measurements did not know the clinical information of the subjects.
Statistical Analysis
Continuous data were presented as mean ± standard deviation (SD), while the categorical data were expressed as frequency. After the normal distributions were tested, comparisons of variables with normal distributions such as endostatin, FEV1/FVC, age, body mass index (BMI) and Low Density Lipoprotein (LDL) were analyzed by one-way analysis of variance. Comparisons of parameters with skewed distributions such as CRP, VEGF, smoking pack, forced expiratory volume in 1 s % predicted (FEV1%pre), maximal expiratory flow (MEF) 75, MEF50, MEF25, MEF75/25 and white blood cells (WBC) were analyzed by Kruskal–Wallis test and Mann–Whitney test. Differences between sex and smoking status were analyzed by Chi-square tests. Pearson’s correlation coefficient and Spearman’s rho were used for correlation analyses between endostatin with lung function values and other inflammatory markers. Binary logistic regression analyses were used for multivariate analysis to assess which background variables were predictive factors for COPD, and odds ratios were calculated with a 95% confidence interval (CI). SPSS 25.0 for Windows (IBM, Chicago, IL, USA) was used for all the data analyses. Significance was defined with a P-value of 0.05. Pictures were made with Graphpad Prism7.0 (GraphPad Software, San Diego, CA, USA).
Results
Characteristics of Subjects
All the demographic data and clinical characteristics (including smoking status, BMI, lung function, circulatory endostatin and CRP levels) of the subjects are summarized in Table 1. In the stable COPD patient group, 11 patients were categorized as GOLD 1, 42 as GOLD 2, 31 as GOLD 3 and 16 as GOLD 4 based on the GOLD criteria. As expected, FEV1/FVC ratio, FEV1%pre and parameters of small airway obstruction (SAO) such as MEF50, MEF25 and MEF75/25 were significantly lower in COPD patients in comparison with the healthy group. Meanwhile, age, WBC, CRP, VEGF and endostatin was higher in the COPD group, and even more in the AECOPD group.
Table 1.
Characteristics of Patients with COPD and Healthy Subjects
| Variables | Heathy Controls (n=68) | Stable COPD (n=100) | AECOPD (n=130) | P |
|---|---|---|---|---|
| Sex (male/female) | 38/30 | 78/22 | 95/35 | 0.006a |
| Age | 52.85±14.22 | 63.34±9.94 | 71.28±9.94 | <0.001 |
| BMI | 24.91±3.86 | 23.14±3.01 | 23.07±3.57 | 0.001b |
| Smoking status(S/NS) | 23/45 | 76/24 | 89/41 | <0.001a |
| Pack-years for ever-smokers | 33.53±35.77 | 38.96±24.08 | 35.70±24.70 | 0.134 |
| FEV1/FVC, % | 86.18±7.44 | 49.73±12.41 | <0.001b | |
| FEV1%pre | 102.50±17.95 | 53.17±21.52 | <0.001 | |
| MEF75 | 85.84±23.55 | 27.07±19.89 | <0.001 | |
| MEF50 | 91.59±28.71 | 17.52±12.08 | <0.001 | |
| MEF25 | 97.76±42.92 | 17.06±11.21 | <0.001 | |
| MEF75/25 | 88.83±29.15 | 16.40±10.61 | <0.001 | |
| CAT | 4.31±6.57 | 14.63±6.58 | <0.001 | |
| WBC, 109/L | 5.64±1.57 | 6.37±1.85 | 7.74±3.43 | <0.001 |
| VEGF (pg/mL) | 91.62±57.87 | 115.22±87.13 | 144.00±99.54 | <0.001 |
| CRP (ug/mL) | 1.50±1.67 | 3.56±4.96 | 3.79±2.23 | <0.001 |
| LDL (mmo/L) | 2.40±0.70 | 2.03±0.84 | 2.29±0.77 | 0.007b |
| Endostatin(ng/mL) | 68.86±29.17 | 89.29±30.33 | 102.09±36.59 | <0.001b |
Notes: Continuous data were presented as mean ± standard deviation (SD), whereas categorical data were expressed as frequency. aχ2 test was used to test the significance of the difference in sex proportions and smoking status. aThe data were analyzed by Chi-square tests. bThe data were analyzed by one-way analysis of variance.
Abbreviations: COPD, chronic obstructive pulmonary disease; AECOPD, acute exacerbation of COPD; BMI, body mass index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; MEF75, maximum expiratory flow at 75%; MEF50, maximum expiratory flow at 50%; MEF25, maximum expiratory flow at 25%; WBC, white blood cells; CRP, C-reactive protein; VEGF, vascular endothelial growth factor; LDL, Low-Density Lipoprotein.
Circulating Endostatin in COPD and Healthy Control
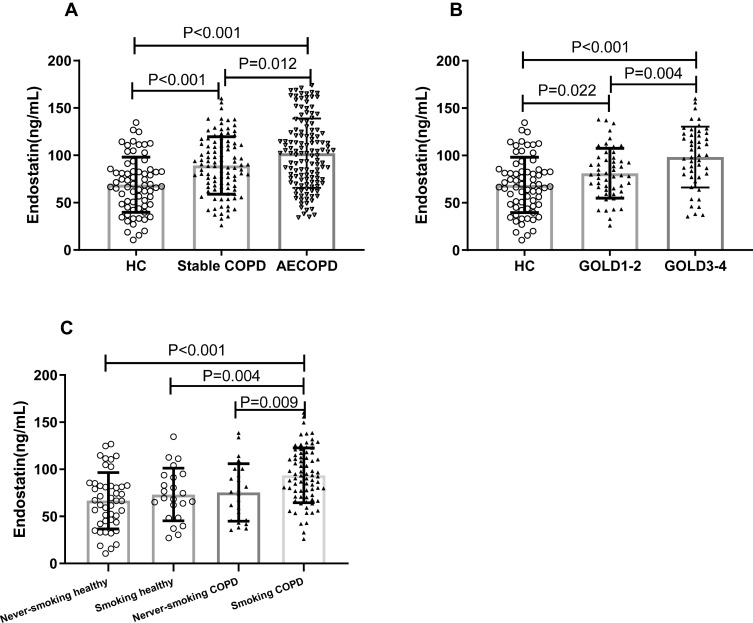
As shown in Figure 1A, serum endostatin was notably more in stable COPD group compared to healthy control, and AECOPD had a higher level than stable COPD patients. In addition, within the stable COPD group, we found the level of serum endostatin is linked to the severity of COPD, as it was higher in GOLD 3–4 compared to GOLD 1–2 (Figure 1B). Furthermore, we found COPD patients with smoking history had a higher level of serum endostatin than the patients who had never smoked (Figure 1C).
Figure 1.
Comparison of serum endostatin levels among different groups. Serum endostatin levels showed an upward trend in COPD patients (A, B); differences of endostatin levels between ever-smoking and never smoking were observed in stable COPD group but not in the healthy control group (C).
Abbreviations: HC, healthy control; COPD, chronic obstructive pulmonary disease; AECOPD, COPD with acute exacerbation; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Correlation of Serum Endostatin with Risk of Exacerbation
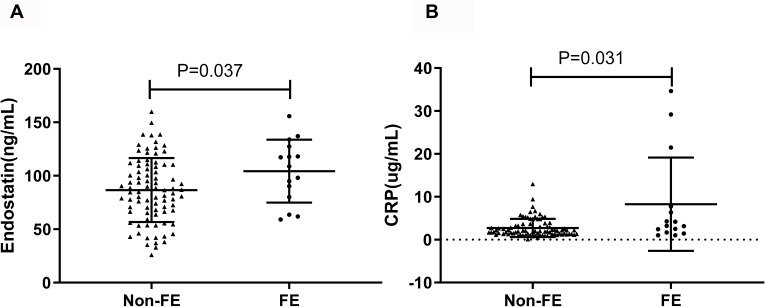
In order to deeply analyze the correlation between circulating endostatin and exacerbation, we compared the levels of serum endostatin and CRP in stable COPD subjects with frequent exacerbation (≥2 exacerbations per year) to those in patients with less exacerbation in the past 1 year. As shown in Figure 2, higher concentrations of circulating CRP and endostatin were found in COPD patients with frequent exacerbations compared to the patients with less exacerbation.
Figure 2.
Comparison of serum endostatin and CRP between different stable COPD patients. Circulating endostatin (A) and CRP (B) were significantly higher in the FE group compared to the non-FE group.
Abbreviations: FE, frequently exacerbation; CRP, C-reactive protein.
Correlation Between Endostatin and Lung Function
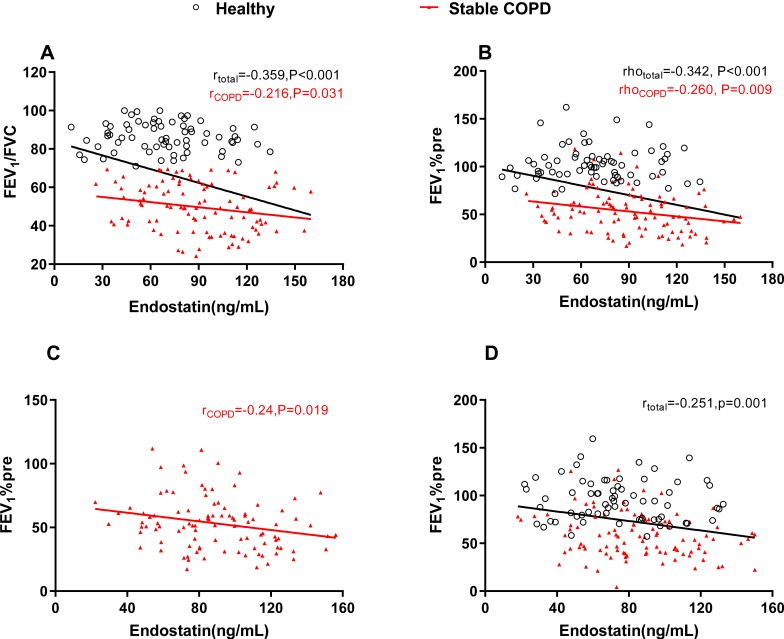
Because FEV1/FVC is normal distribution data, we analyzed the correlation between endostatin and FEV1/FVC by Pearson’s correlation coefficient test. Spearman’s rho was used for analyzing the correlation of endostatin with FEV1%pre, MEF75, MEF50 MEF25 and MEF75/25 as they were skew distribution. Our data showed that serum endostatin was negatively correlated to FEV1/FVC, FEV1%pre, MEF75, MEF50 and MEF75/25 no matter in all the healthy and stable COPD subjects or in the stable COPD group (Figure 3A and B, Table 2). Due to the differences of age, BMI, sex and smoking status between healthy people and COPD patients, partial correlations were used to analyze the correlation between endostatin and pulmonary function parameters by adjusting age, BMI, sex and smoking status (Table 2). We found endostatin level was still inversely associated with FEV1%pre after adjustment (r=−0.24, P=0.019 for stable COPD; r=−0.251, P=0.001 for a total of healthy control and stable COPD) (Figure 3C and D).
Figure 3.
Serum endostatin was correlated with pulmonary function. Serum endostatin was inversely correlated with (A) FEV1/FVC and (B) FEV1%pre. After adjusting age, BMI, sex and smoking status, endostatin level was still inversely associated with FEV1%pre in the stable COPD group (C) and in the total of healthy control and stable COPD (D). The red solid line denotes the line of best fit in stable COPD, the black line represents the line of best fit in a total of healthy control and stable COPD.
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
Table 2.
Correlations of Endostatin with Lung Function in Stable COPD and Healthy Control
| Linear Correlation | Partial Correlation | |||
|---|---|---|---|---|
| Stable COPD | HC and Stable COPD | Stable COPD | HC and Stable COPD | |
| FEV1/FVC | r=−0.216, P=0.031 |
r=−0.359, P<0.001 |
r=−0.181 P=0.078 |
r=−0.268 P=0.001 |
| FEV1%pre | rho=−0.260, P=0.009 |
rho=−0.342, P<0.001 |
r=−0.240 P=0.019 |
r=−0.251 P=0.001 |
| MEF75 | rho=−0.275, P=0.007 |
rho=−0.310, P<0.001 |
r=−0.193 P=0.069 |
r=−0.194 P=0.015 |
| MEF50 | rho=−0.257, P=0.010 |
rho=−0.328, P<0.001 |
r=−0.216 P=0.041 |
r=−0.178 P=0.026 |
| MEF25 | rho=−0.184, P=0.067 |
rho=−0.312, P<0.001 |
r=−0.201 P=0.058 |
r=−0.173 P=0.031 |
| MEF75/25 | rho=−0.257, P=0.010 |
rho=−0.305, P<0.001 |
r=−0.249 P=0.018 |
r=−0.161 P=0.045 |
Notes: Serum endostatin was negatively correlated to FEV1/FVC, FEV1%pre, MEF75, MEF50, and MEF75/25 no matter in all the healthy subjects and stable COPD or only the stable COPD group. Endostatin correlated to MEF25 only in the total group of healthy control and stable COPD. After adjusting age, sex, BMI and smoking status, serum endostatin was still negatively with FEV1%pre, MEF50, and MEF75/25 in the stable COPD group. Bold text means significance.
Abbreviations: HC, healthy controls; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; MEF75, maximum expiratory flow at 75%; MEF50, maximum expiratory flow at 50%; MEF25, maximum expiratory flow at 25%.
Correlation Between Endostatin and Other Serum Markers
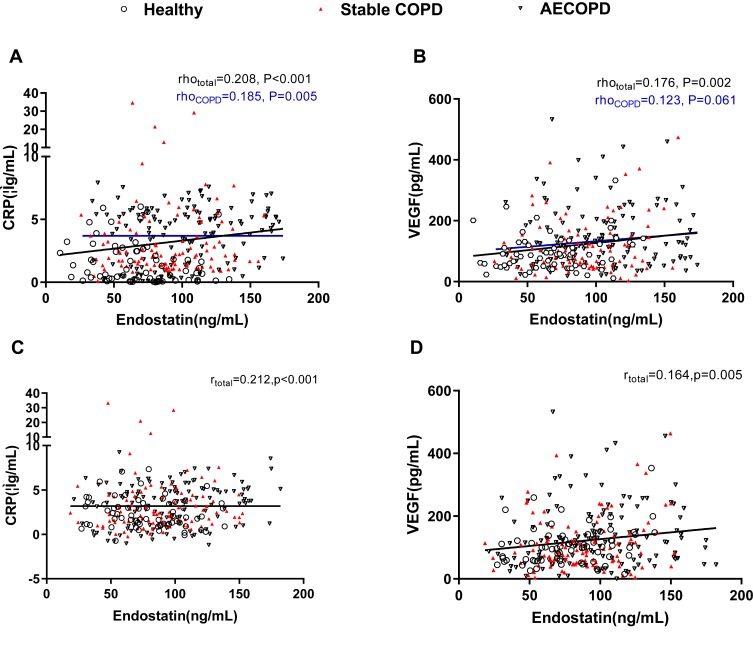
We further analyzed the correlation between endostatin and other serum markers, such as CRP and VEGF. Endostatin was positively correlated to CRP in the COPD group (including stable and AECOPD) (rho=0.185, P= 0.005) and all the subjects (rho=0.208, P<0.001) (Figure 4A). However, endostatin was only associated with VEGF in the total participants (rho=0.176, P=0.002), not in the COPD group (P=0.061) (Figure 4B). Similarly, we also analyzed the partial correlations between endostatin with CRP and VEGF by adjusting age, BMI, sex and smoking status. Then, we found that endostatin level was positively associated with CRP and VEGF in all the subjects (r=0.212, P<0.001 for CRP; r=0.164, P=0.005 for VEGF) after adjustment (Figure 4C and D).
Figure 4.
Correlation analyses between serum endostatin levels and other COPD biomarkers. Serum endostatin was positively correlated to serum CRP (A) and VEGF (B). After adjusting age, BMI, sex and smoking status, endostatin was positively associated with CRP (C) and VEGF (D) in all the participants. The blue solid line denotes the line of best fit in COPD, the black line represents the line of best fit in all the participants.
Abbreviations: COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; VEGF, vascular endothelial growth factor.
Diagnostic Value of Endostatin for COPD
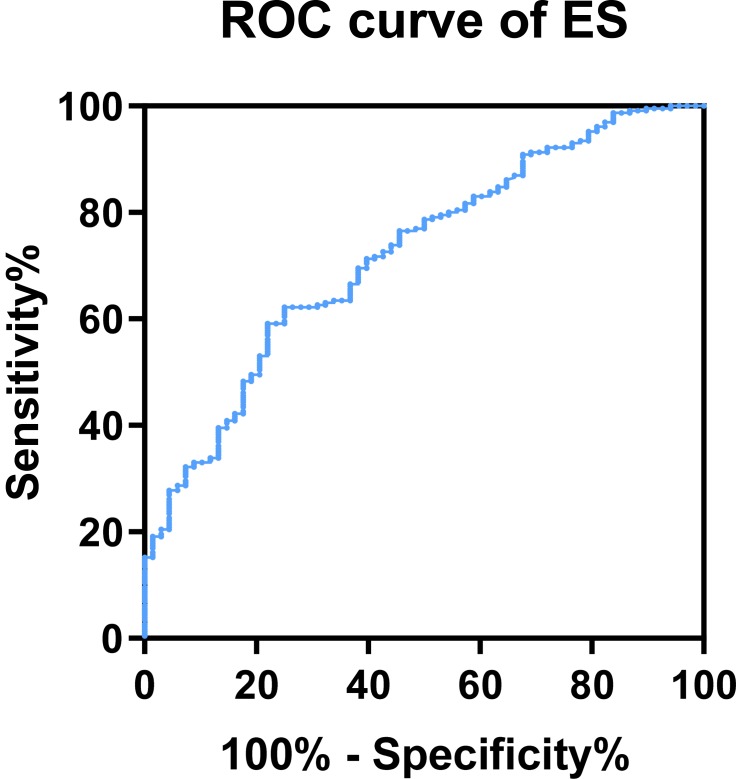
Because endostatin had a remarkably higher concentration in the serum of COPD patients, and was negatively correlated to lower lung function, we further investigated the possible merit of serum endostatin in diagnosing COPD. Receiver operating characteristic (ROC) curve analysis indicated that the area under curve (AUC) of endostatin to distinguish COPD was 0.72 (95% CI, 0.654–0.787) (Figure 5). With a cut-off value of 83.27 ng/mL, the sensitivity was 62.2% and the specificity was 75.0%, respectively.
Figure 5.
Diagnostic value of endostatin for COPD.
Prediction of Endostatin on COPD
Binary logistic regression analysis was used to calculate the odds ratios to test the association of endostatin with COPD. Sex, age, smoking condition, BMI and serum CRP were also included in this model because they were different between the healthy control and all the COPD patients. We found age, smoking condition, BMI, serum CRP and endostatin were all associated with COPD prevalence. Serum endostatin in all the subjects were grouped into 4 quartiles: quartile 1 ranged less than 51.00ng/mL, quartiles 2 ranged from 51.00ng/mL to 91.00ng/mL, quartile 3 ranged from 91.00ng/mL to 131ng/mL and quartile 4 ranged more than 131ng/mL. The odds ratio was 1.994 (95% CI: 1.253, 3.175) per 40ng/mL (Table 3).
Table 3.
Binary logistic regression analysis on the prediction of COPD
| Risk Factor | COPD | Odds Ratio(95%CI) | P value |
|---|---|---|---|
| Sex | 0.106 | ||
| Female | 59/87(67.8) | 1(Reference) | |
| Male | 185/211(87.6) | 0.430(0.154, 1.198) | |
| Age | 1.081 (1.048, 1.114) | <0.001 | |
| Smoking condition | |||
| Smokers | 177/188 (94.1) | 1 (Reference) | |
| Never smokers | 67/110 (60.9) | 0.182 (0.067, 0.491) | <0.001 |
| BMI | 0.892 (0.802, 0.992) | 0.035 | |
| CRP (ug/mL) | 1.510 (1.218, 1.872) | <0.001 | |
| Endostatin (ng/mL) | 1.994 (1.253, 3.175) Per 40 ng/mL | 0.004 | |
| ≤51. 00 | 21/40 (52.5) | ||
| 51.01–91.00 | 91/121(75.2) | ||
| 91.01–131.00 | 93/98(94.9) | ||
| ≥131.00 | 39/39(100) |
Notes: Sex, age, smoking condition, BMI, serum CRP were also included in this model. Age, smoking condition, BMI, serum CRP and endostatin were all associated with COPD prevalence. The odds ratio of serum endostatin was 1.994 (95%CI: 1.253, 3.175) per 40ng/mL.
Abbreviations: COPD, chronic obstructive pulmonary disease; BMI, body mass index; CRP, C-reactive protein.
Discussion
This study investigated the difference of circulating endostatin level between healthy people and COPD patients including the patients with acute exacerbation. We demonstrated that serum endostatin level was markedly higher in COPD patients, which was even more in the AECOPD group, and it was statistically associated with the severity of airflow obstruction and SAO since it was reversely correlated to FEV1%pre, MEF50 and MEF75/25. Besides, the history of smoking and exacerbation also affected the concentration of serum endostatin as we showed that in the stable COPD group, patients having frequent exacerbation in the past 1 year had higher level of serum endostatin compared to the patients who had less exacerbation, and patients who were ever or currently smokers had higher level of serum endostatin than the patients who had never smoked. Furthermore, we observed that circulating endostatin was positively related to VEGF and CRP. More importantly, we demonstrated that circulating endostatin was independently associated with morbidity of COPD. Therefore, we provided the first evidence which linked serum endostatin to COPD severity.
Angiogenesis, the formation of new blood vessels, is a critical physiologic and pathologic process in many disease conditions including wound repair, tumor growth, and other chronic diseases.16,17 Recently, studies threw light that angiogenesis also contributed to the progression of COPD because it initiated the remodeling of bronchial vasculature, followed by airway-remodeling during the progression of chronic airway inflammation.18 Even though the molecular mechanisms are still incompletely understood, a series of growth factors and cytokines released from different places of vascular wall and airway may play roles in this.19,20 Among those factors, vascular endothelial growth factor (VEGF) is a very important regulator of angiogenesis and vascular remodeling.21 It functions through binding to two cellular receptors VEGFR1 (Flt-1) and VEGFR2/KDR (Flk-1), on endothelial cells.22 It was declaimed that VEGF increased in the airways and serum of patients with COPD, which identified the patients with poorer prognosis.22,23 In this study, we showed that serum VEGF level increased in COPD patients, being consistent with the previous reports. Endostatin, a 20-kDa peptide derived from the carboxy terminus of collagen XVIII, is a potent inhibitor of angiogenesis and interferes with VEGF/VEGFR signaling.8,24 It has been reported that endostatin was involved in the pathogenesis of tumors, arthritis, heart failure, chronic kidney disease and pulmonary hypertension and circulatory of endostatin were increased in patients with those diseases.7,12,14,25–27 Additionally, the imbalance between vascular endothelial growth factor and endostatin levels in induced sputum from emphysema patients was reported before, which is the only study connecting endostatin with COPD.15 Thus, it is necessary to conduct more research to explore the role of endostatin in COPD.
In this study, we found a significantly elevated concentration of serum endostatin in patients with COPD, which suggested a potential role of endostatin in COPD. Even currently we do not know the exact mechanisms, there can be some speculations. Firstly, other risk factors that were closely related to endostatin, such as age and obesity, might contribute to the adverse effects associated with increased endostatin levels because they differed between healthy people and COPD patients in our study. Secondly, different gender and smoking condition between healthy and COPD in our study could also lead to the difference of serum endostatin level. However, endostatin was still associated with lung function after we adjusted for these parameters, indicating that endostatin might play a direct role. Besides, pulmonary vascular endothelial dysfunction in COPD might be a more important explanation. Damage and proliferation of endothelial cells (EC) and pulmonary microvascular endothelial dysfunction including increased vascular permeability, vasodilation and angiogenesis trigger remodeling of the pulmonary vasculature, thus inducing pulmonary hypertension (PH), which is a characteristic of COPD.28,29 Apoptosis of pulmonary endothelial cells was also involved in the pathogenesis of COPD by causing emphysema.30 Endostatin is an effective inhibitor of endothelial cells, which can inhibit the functions of not only endothelial cells but also endothelial progenitor cells and induce apoptosis of these cells.31,32 Therefore, we speculated that elevated endostatin might be one of the mechanisms for the endothelial apoptosis and endothelial dysfunction in COPD patients.
When a patient with COPD has a sustained (e.g. 24–48 hrs) increase in cough, expectoration and/or dyspnea, the patient will be diagnosed as an acute exacerbation of chronic obstructive pulmonary disease (AECOPD), which to a very large extent, increases the frequency of visits to the emergency department or hospitalizations and expands healthcare costs. Previous studies indicated that cardiac comorbidity was the most common cause of AECOPD and attenuated endothelial repair might cause an increased risk of cardiovascular events in patients with COPD.3,33 In this study, we found that COPD patients with more exacerbation had a higher level of endostatin in circulation compared to the patients with less exacerbation, which linked the endothelial dysfunction with AECOPD again and could predict the risk of cardiac diseases in COPD.
In addition, we observed that the serum endostatin level was particularly higher in GOLD 3–4 COPD patients than GOLD 1–2 patients, signifying that circulating endostatin may be negatively associated with the severity of COPD. The correlation analyses between endostatin level and lung function parameters further validated this hypothesis by showing that serum endostatin was inversely correlated to FEV1/FVC, FEV1%pre and SAO parameters. Although the underlying mechanisms on how endostatin plays a role in the airflow obstruction could not be elucidated now, airway angiogenesis and endothelial dysfunction could be the potential mechanisms for this, which needs studying further. Finally, we analyzed the diagnostic and predictive role of endostatin by analyzing the ROC curve and binary logistic regression and found that endostatin could be a diagnostic marker for COPD, suggesting endostatin is worthy to study in the future.
Our research aimed to explore whether endostatin had a relationship with COPD and the results showed that serum endostatin level was significantly higher in COPD patients and correlated to lung function and exacerbation. However, there were also some limitations in this study. Firstly, only 100 stable COPD patients without computed tomography data were recruited, limiting our analyses on the correlation between endostatin with lung function and systemic inflammation in different phenotypes of COPD. Secondly, it was difficult to conclude the causality between endostatin or other parameters with COPD because this study was a cross-sectional study and a retrospective analysis, which needs more prospective or in vitro studies to clarify the possible mechanisms under these observations. Moreover, we only detected CPR and VEGF besides endostatin, which narrowed the investigation of the relationship between endostatin with inflammatory response and airway angiogenesis in COPD. Lastly, several other samples that can better reflect the condition of airways, such as bronchoalveolar lavage fluid (BALF) and sputum, should also be analyzed in the future research.
Collectively, the present study is the first demonstration that serum endostatin is a novel biomarker of COPD, which is associated with lung function and airway angiogenesis. It may be useful for diagnosing disease and predicting the exacerbation of disease. This study also provides a new idea to further understand the mechanisms for the pathogenesis and development of COPD. However, further prospective research or mechanical studies need conducting to explain the specific role of endostatin in the pathogenesis of COPD.
Acknowledgments
This work was supported by the National Key Research and Development Program (2016YFC0903600), National Natural Science Foundation of China (31871157, 81830001, 81670038, and 81700036), and Health and Family Planning Commission of Sichuan Province (17PJ009). The funders had no role in study design, data collection or analysis, decision to publish, and manuscript preparation. The abstract of this paper was presented at the ERS INTERNATIONAL CONGRESS 2019 as a poster presentation with interim findings. The poster’s abstract was published in ‘Poster Abstracts’ in European Respiratory Journal: https://erj.ersjournals.com/content/54/suppl_63/PA1717.
Author Contributions
All authors contributed toward data collection, statistical analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- 1.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agusti AG, Noguera A, Sauleda J, et al. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:347–360. doi: 10.1183/09031936.03.00405703 [DOI] [PubMed] [Google Scholar]
- 4.Peinado VI, Barbera JA, Ramirez J, et al. Endothelial dysfunction in pulmonary arteries of patients with mild COPD. Am J Physiol. 1998;274:L908–L913. doi: 10.1152/ajplung.1998.274.6.L908 [DOI] [PubMed] [Google Scholar]
- 5.Eid AA, Ionescu AA, Nixon LS, et al. Inflammatory response and body composition in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1414–1418. doi: 10.1164/ajrccm.164.8.2008109 [DOI] [PubMed] [Google Scholar]
- 6.Pihtili A, Bingol Z, Kiyan E. Serum endocan levels in patients with stable COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3367–3372. doi: 10.2147/COPD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/S0092-8674(00)81848-6 [DOI] [PubMed] [Google Scholar]
- 8.Wickstrom SA, Veikkola T, Rehn M, et al. Endostatin-induced modulation of plasminogen activation with concomitant loss of focal adhesions and actin stress fibers in cultured human endothelial cells. Cancer Res. 2001;61:6511–6516. [PubMed] [Google Scholar]
- 9.Abdollahi A, Hahnfeldt P, Maercker C, et al. Endostatin’s antiangiogenic signaling network. Mol Cell. 2004;13:649–663. doi: 10.1016/S1097-2765(04)00102-9 [DOI] [PubMed] [Google Scholar]
- 10.Schmidt A, Addicks K, Bloch W. Opposite effects of endostatin on different endothelial cells. Cancer Biol Ther. 2004;3:1162–1166; discussion 1167–1168. doi: 10.4161/cbt.3.11.1219 [DOI] [PubMed] [Google Scholar]
- 11.Ruge T, Carlsson AC, Larsson TE, et al. Endostatin level is associated with kidney injury in the elderly: findings from two community-based cohorts. Am J Nephrol. 2014;40:417–424. doi: 10.1159/000369076 [DOI] [PubMed] [Google Scholar]
- 12.Hebbar M, Peyrat JP, Hornez L, et al. Increased concentrations of the circulating angiogenesis inhibitor endostatin in patients with systemic sclerosis. Arthritis Rheum. 2000;43:889–893. doi: [DOI] [PubMed] [Google Scholar]
- 13.Funatsu H, Yamashita H, Noma H, et al. Stimulation and inhibition of angiogenesis in diabetic retinopathy. Jpn J Ophthalmol. 2001;45:577–584. doi: 10.1016/S0021-5155(01)00420-8 [DOI] [PubMed] [Google Scholar]
- 14.Damico R, Kolb TM, Valera L, et al. Serum endostatin is a genetically determined predictor of survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;191:208–218. doi: 10.1164/rccm.201409-1742OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanazawa H, Hirata K, Yoshikawa J. Imbalance between vascular endothelial growth factor and endostatin in emphysema. Eur Respir J. 2003;22:609–612. doi: 10.1183/09031936.03.00030403 [DOI] [PubMed] [Google Scholar]
- 16.Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235:442–447. doi: 10.1126/science.2432664 [DOI] [PubMed] [Google Scholar]
- 17.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287 [DOI] [PubMed] [Google Scholar]
- 18.Alagappan VK, De Boer WI, Misra VK, Mooi WJ, Sharma HS. Angiogenesis and vascular remodeling in chronic airway diseases. Cell Biochem Biophys. 2013;67:219–234. doi: 10.1007/s12013-013-9713-6 [DOI] [PubMed] [Google Scholar]
- 19.De Boer WI, Hau CM, Van Schadewijk A, et al. Expression of epidermal growth factors and their receptors in the bronchial epithelium of subjects with chronic obstructive pulmonary disease. Am J Clin Pathol. 2006;125:184–192. doi: 10.1309/W1AXKGT7UA37X257 [DOI] [PubMed] [Google Scholar]
- 20.Ganesan S, Unger BL, Comstock AT, et al. Aberrantly activated EGFR contributes to enhanced IL-8 expression in COPD airways epithelial cells via regulation of nuclear FoxO3A. Thorax. 2013;68:131–141. doi: 10.1136/thoraxjnl-2012-201719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer N, Akdis CA. Vascular endothelial growth factor as a key inducer of angiogenesis in the asthmatic airways. Curr Allergy Asthma Rep. 2013;13:1–9. doi: 10.1007/s11882-012-0317-9 [DOI] [PubMed] [Google Scholar]
- 22.Kranenburg AR, De Boer WI, Alagappan VK, Sterk PJ, Sharma HS. Enhanced bronchial expression of vascular endothelial growth factor and receptors (Flk-1 and Flt-1) in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:106–113. doi: 10.1136/thx.2004.023986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farid Hosseini R, Jabbari Azad F, Yousefzadeh H, et al. Serum levels of vascular endothelial growth factor in chronic obstructive pulmonary disease. Med J Islam Repub Iran. 2014;28:85. [PMC free article] [PubMed] [Google Scholar]
- 24.Hajitou A, Grignet C, Devy L, et al. The antitumoral effect of endostatin and angiostatin is associated with a down-regulation of vascular endothelial growth factor expression in tumor cells. FASEB J. 2002;16:1802–1804. [DOI] [PubMed] [Google Scholar]
- 25.Nagashima M, Asano G, Yoshino S. Imbalance in production between vascular endothelial growth factor and endostatin in patients with rheumatoid arthritis. J Rheumatol. 2000;27:2339–2342. [PubMed] [Google Scholar]
- 26.Kobusiak-Prokopowicz M, Jolda-Mydlowska B, Grzebieniak T, Poczatek K, Mysiak A. Expression of proinflammatory factors, proangiogenic factors and endostatin in patients with heart failure and different grades of collateral circulation development. Adv Clin Exp Med. 2015;24:987–994. doi: 10.17219/acem/33811 [DOI] [PubMed] [Google Scholar]
- 27.Kanbay M, Afsar B, Siriopol D, et al. Endostatin in chronic kidney disease: associations with inflammation, vascular abnormalities, cardiovascular events and survival. Eur J Intern Med. 2016;33:81–87. doi: 10.1016/j.ejim.2016.06.033 [DOI] [PubMed] [Google Scholar]
- 28.Koyama S, Sato E, Tsukadaira A, et al. Vascular endothelial growth factor mRNA and protein expression in airway epithelial cell lines in vitro. Eur Respir J. 2002;20:1449–1456. doi: 10.1183/09031936.02.00089802 [DOI] [PubMed] [Google Scholar]
- 29.Arao T, Takabatake N, Sata M, et al. In vivo evidence of endothelial injury in chronic obstructive pulmonary disease by lung scintigraphic assessment of (123)I-metaiodobenzylguanidine. J Nucl Med. 2003;44:1747–1754. [PubMed] [Google Scholar]
- 30.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106:1311–1319. doi: 10.1172/JCI10259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin CH, Chen J, Ziman B, et al. Endostatin and kidney fibrosis in aging: a case for antagonistic pleiotropy? Am J Physiol Heart Circ Physiol. 2014;306:H1692–H1699. doi: 10.1152/ajplegacy.1975.229.3.570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YM, Hwang S, Kim YM, et al. Endostatin blocks vascular endothelial growth factor-mediated signaling via direct interaction with KDR/Flk-1. J Biol Chem. 2002;277:27872–27879. doi: 10.1074/jbc.M202771200 [DOI] [PubMed] [Google Scholar]
- 33.Decramer M, De Benedetto F, Del Ponte A, Marinari S. Systemic effects of COPD. Respir Med. 2005;99(Suppl B):S3–10. doi: 10.1016/j.rmed.2005.09.010 [DOI] [PubMed] [Google Scholar]