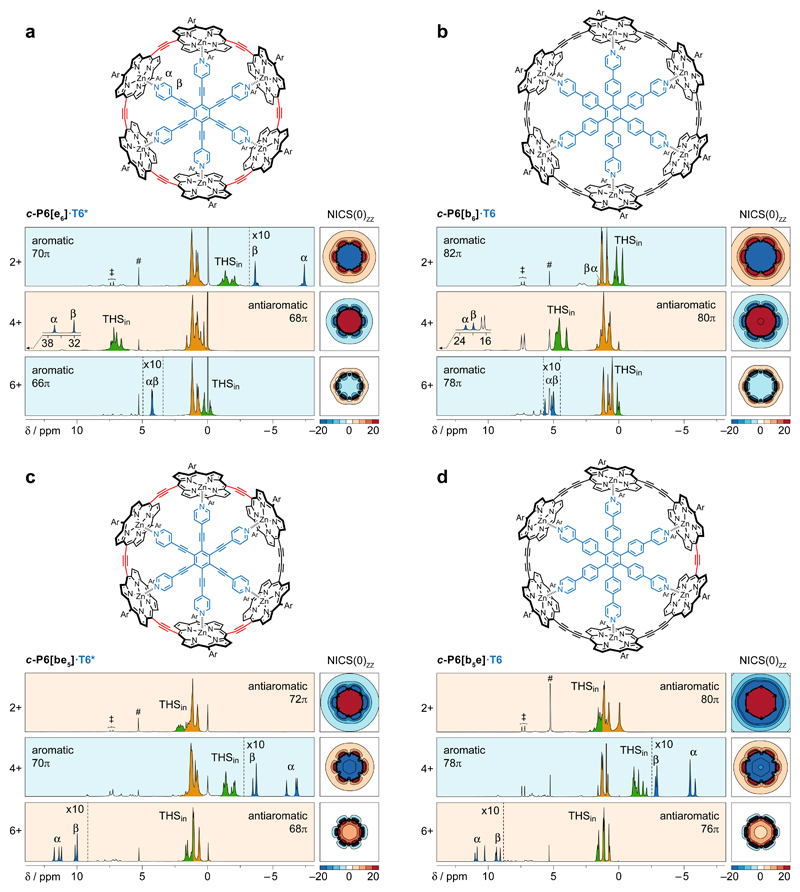
Fig. 1. 1H NMR spectra of the aromatic and antiaromatic six-porphyrin nanoring template complexes in oxidation states 2+, 4+ and 6+.
a, c-P6[e6]·T6*; b, c-P6[be5]·T6*; c, c-P6[b5e]·T6 and d, c-P6[b6]·T6. A grid plot of the NICS(0)zz value in the xy plane of the nanoring, calculated without template, is shown for each oxidation state of each complex (5 × 5 nm; LC-ωhPBE/6-31G*, ω = 0.1; colour axis truncated above 20 and below –20 ppm; contours drawn every 5 ppm). 1H NMR spectra recorded at 500 MHz in CD2Cl2; oxidised states are generated by titration with thianthrenium hexafluoroantimonate. # and ‡ denote CHDCl2 and thianthrene, respectively. Detailed spectra are shown in Supplementary Figs. 9–12 and 19–34. Dashed vertical lines indicate 10-fold magnifications.