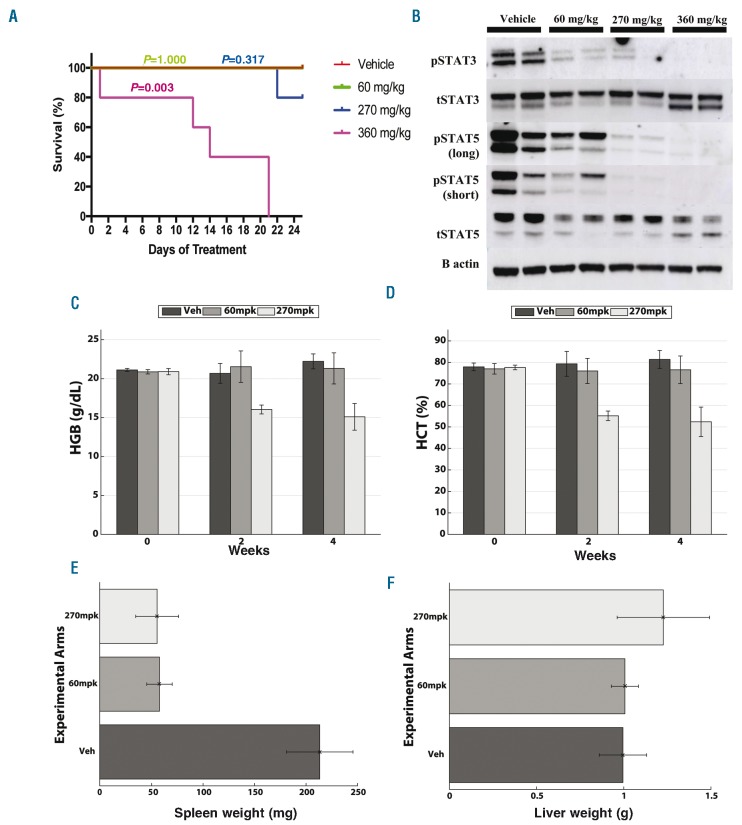
Figure 3.
In vivo data confirm in silico predictions. (A) Kaplan-Meier Survival Plot. Group 1 (red) was treated with vehicle twice daily. Group 2 (green) was treated with 60 mg/kg twice daily. Group 3 (blue) was treated with 270 mg/kg twice daily for five days followed by a two-day holiday. Group 4 (purple) was treated with 360 mg/kg twice daily for three days followed by a four-day holiday. The 360 mg/kg treatment group showed a significant decrease in survival (P=0.003) compared to other experimental arms. (B) Western blot. While mice treated with 60 mg/kg ruxolitinib showed partial pSTAT3 and pSTAT5 signaling, mice treated with 270 mg/kg showed nearly complete inhibition of pSTAT5 signaling, indicating there is improved signaling inhibition with increased dose. Complete blood counts measured before and after treatment revealed significant improvement in (C) hemoglobin and (D) hematocrit in group treated with 270 mg/kg bis in die (BID) (five times/week) in comparison to mice treated chronically with 60 mg/kg BID as well as vehicle starting at two weeks after treatment. A non-parametric Wilcoxon rank sum test revealed differences between the 270 mg/kg group and two others to be significant with P-values <0.016 for both hemoglobin and hematocrit. Complete results from statistical testing can be found in the Online Supplementary Table S1. (E) Mice treated with 270 mg/kg BID five times/week and chronic 60 mg/kg BID ruxolitinib showed marked reduction in spleen weight compared to vehicle treatment at the conclusion of 25 days of treatment (P-values of 0.007 comparing treatment groups to vehicle using a Wilcoxon rank sum test). (F) There was no significant change in liver weight between all groups supporting the absence of drug-related toxicity. All pairwise statistical test results for all three groups for liver and spleen can be found in Online Supplementary Table S1.