Abstract
A number of effective drugs have been developed through animal experiments, contributing to the health of many patients. In particular, the WHHL rabbit family (WHHL rabbits and its advanced strains (coronary atherosclerosis-prone WHHL-CA rabbits and myocardial infarction-prone WHHLMI rabbits) developed at Kobe University (Kobe, Japan) contributed greatly in the development of cholesterol-lowering agents. The WHHL rabbit family is animal models for human familial hypercholesterolemia, coronary atherosclerosis, and coronary heart disease. At the end of breeding of the WHHL rabbit family, this review summarizes the contribution of the WHHL rabbit family to the development of lipid-lowering agents and anti-atherosclerosis agents. Studies using the WHHL rabbit family demonstrated, for the first time in the world, that lowering serum cholesterol levels or preventing LDL oxidation can suppress the progression and destabilization of coronary lesions. In addition, the WHHL rabbit family contributed to the development of various compounds that exhibit lipid-lowering and anti-atherosclerotic effects and has also been used in studies of gene therapeutics. Furthermore, this review also discusses the causes of the increased discrepancy in drug development between the results of animal experiments and clinical studies, which became a problem in recent years, and addresses the importance of the selection of appropriate animal models used in studies in addition to an appropriate study design.
Keywords: Anti-atherosclerotic agents, Cholesterol-lowering agents, Development of therapeutics, Species differences, WHHL rabbit
Introduction
According to a report by WHO (https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death), out of 56.9 million deaths world-wide in 2016, more than 9 million were due to ischemic heart disease and increased by approximately 2 million since 2000. It has long been pointed out that hypercholesterolemia is associated with the onset of ischemic heart disease. Animal models for human disease greatly contributed to the development or validation of therapeutic agents or devices and diagnostic equipment or technologies. In particular, the Watanabe heritable hyperlipidemic (WHHL) rabbit, an animal model of familial hypercholesterolemia1–3), and its advanced strains developed by selective breeding, the WHHL-CA rabbits (provisional name)4, 5) showing spontaneous coronary atherosclerosis, and the WHHLMI rabbit showing spontaneous coronary atherosclerosis and myocardial infarction6) played an important role in the development and validation of therapeutic agents for hypercholesterolemia or atherosclerosis. These rabbit strains were developed at Kobe University (Kobe, Japan). In this review, WHHL rabbits, WHHL-CA rabbits, and WHHLMI rabbits are referred to as the WHHL rabbit family. However, breeding of the WHHL rabbit family at Kobe University ended in June 2018. Currently, a few institutes are trying to breed WHHLMI rabbits. On this occasion, the contribution of the WHHL rabbit family in the development or validation of therapeutic agents and diagnostic equipment was summarized in this review. This review also addressed the issues of increasing discrepancies in drug development between the results of animal experiments and clinical studies. The history of the development of the WHHL rabbit family, the characteristics, and their contribution to studies on lipoprotein metabolism and atherosclerosis are described in the Part I7). In this review, terms “WHHL rabbit”, “WHHL-CA rabbits”, and “WHHLMI rabbit” indicate the homozygotes.
Contribution of the WHHL Rabbit Family to the Development of Therapeutic Agents and Imaging Technology for Atherosclerosis
Studies using the WHHL rabbit family that examined lipid-lowering effects and/or anti-atherosclerotic effects of compounds, foods, proteins, and gene therapy are summarized in Table 1.
Table 1. Studies using the WHHL rabbit family to develop compounds with lipid-lowering effects and anti-atherosclerotic effects.
| Lipid-lowering effects |
Anti-atherosclerosis |
|||
|---|---|---|---|---|
| Cholesterol | Triglyceride | Aorta | Coronary | |
| Statin | ○ | ○ × | ○ × | ○ |
| Anion resin | ○ | × | ○ | n.e. |
| Statin+resin | ○ | × | ○ | ○ |
| Squalene synthase inhibitor | ○ | ○ | ○ | ○ |
| MTP inhibitor | ○ | ○ | n.e. | n.e. |
| ACAT inhibitor | ○ × | × | ○ × | ○ × |
| Fish oil or ω3 fatty acids | ○ | ○ | ○ × | n.e. |
| ApoE | ○ | × | ○ | n.e. |
| Fibrate | × | × | ○ x | n.e. |
| D-47 | ○ | ○ | n.e. | n.e. |
| Probucol | ○ | × | ○ | ○ |
| M-CSF and GM-CSF | ○ | ○ | ○ | n.e. |
| Thiazolidinedione | × | × | × | × |
| Thiazolidinedione+statin | ○ | × | ○ | ○ |
| Ca2+ antagonists | × | × | × | × |
| β-blockers | × | × | × | × |
| Angiotensin converting enzyme inhibitors | × | × | ○ | n.e. |
| Angiotensin-II type I receptor antagonists | × | × | ○ | n.e. |
| LDLR gene therapy | ○ | ○ × | ○ | n.e. |
| Apobec-1 expression in liver | ○ | ○ × | n.e. | n.e. |
| LPL expression in whole body cells | ○ | ○ | × | n.e. |
○, effective; ×, no effect; n.e., not examined. References for studies about lipid-lowering or anti-atherosclerotic effects of each agent, compound or molecule are listed on the WHHL rabbit website (http://www.med.akita-u.ac.jp/~doubutu/WHHL/w-index.html).
Contribution of the WHHL Family to the Establishment of Statins' Lipid-Lowering Effects and Anti-Atherosclerosis Effects
Nowadays, the first-choice medicine for hypercholesterolemia is statins, which are competitive inhibitors of 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase, a rate limiting enzyme in the early stage of cholesterol biosynthesis. The first statin, compactin, was developed by Akira Endo (Sankyo Co., Ltd., Tokyo, Japan) in 1973 8, 9). Since compactin results in no lipid-lowering effect in mice and rats, the research group considered discontinuing the development9). However, compactin continued to be developed because it showed a potent cholesterol-lowering effect in other animals9). The continued development of compactin led to the development of seven statins, which are currently available. The compactin research group considered that it is important that cholesterol-lowering agents should be effective in animal models with spontaneous hypercholesterolemia. In 1980, cholesterol-lowering effects of compactin were examined in WHHL rabbits (homozygotes), and the serum cholesterol levels were decreased dose-dependently10). After cessation in compactin development, pravastatin, a compactin metabolite, was developed11), and pravastatin also lowered serum cholesterol levels dose-dependently in WHHL rabbits11). In addition to compactin and pravastatin, various statins, such as lovastatin12), simvastatin13), fluvastatin14), cerivastatin15), pitavastatin16), and atorvastatin17), also showed potent serum cholesterol-lowering effects in the WHHL rabbit family. The cholesterol-lowering effects of statins are considered to be due to an increase in the number of LDL receptors in the liver. The hepatic LDL receptor activity in WHHL rabbits (homozygote) was increased 11.2-fold with pravastatin treatment18), and similar results were observed in fluvastatin19). Mutant LDL receptor proteins of WHHL fibroblasts were biosynthesized similar to a variant of human familial hypercholesterolemia20), and serum LDL cholesterol levels decrease by approximately 80% at the end of pregnancy in WHHL rabbits21), similar to another variant of human familial hypercholesterolemia22). In that paper, the patient was considered as a heterozygote but was diagnosed as a homozygote in the later examination (personal communication with Kajinami K). These studies suggest that mutant LDL receptors in the WHHL rabbit family may be able to increase, and increased LDL receptors may be able to bind LDL particles. In addition, cholesterol content in newly secreted very low-density lipoprotein (VLDL) was decreased by 18% in pravastatin treated WHHL-CA rabbits23). These studies suggest that statins' cholesterol-lowering effects were due to a dual mechanism, an increase in hepatic LDL receptors, and a decrease in VLDL-cholesterol secretion from liver.
In the original WHHL rabbit strain, atherosclerotic lesions were observed in the aorta but were rare in the coronary arteries1, 4). WHHL-CA rabbits demonstrating coronary atherosclerosis were developed by selective breeding at Kobe University4, 5), and the WHHL-CA rabbit made the possibility to investigate the suppression effect of drugs on coronary lesions exist. Thereafter, anti-atherosclerotic effects of pravastatin were examined using young WHHL-CA rabbits24). Twenty-four weeks of the statin treatment reduced serum cholesterol levels by 28%, and the atherosclerotic lesions were suppressed in the aorta and coronary arteries. This was the first study that statin can suppress coronary atherosclerosis. Since the serum cholesterol levels treated with pravastatin were still high, cholestyramine, a bile acid sequstrant, and pravastatin were co-administered to mature WHHL-CA rabbits aged 10 month for one year to lower serum cholesterol levels to almost normal. These WHHL-CA rabbits had established atherosclerotic lesions. The purpose of this study was to investigate whether atherosclerotic lesions could regress or not25). In the combination treatment, the serum cholesterol levels decreased to 229 ± 23 mg/dl. The degree of atherosclerotic lesions decreased markedly in both coronary arteries and the aorta. However, the degree of atherosclerosis in the combination treatment group (22 months old) was comparable to control rabbits examined at the start of the treatment (10 months old). In the histopathological examination, arterial lesions of the combination treatment group were fibrous lesions rich in collagen fibers and scarce in macrophages and extracellular lipid deposits, whereas arterial lesions in control and placebo groups were atheromatous lesions enriched in macrophages or necrotic cores. These results suggested that cholesterol-lowering therapy can regress atherosclerotic lesions if the lesions are not fibrotic. In the REGRESS study26), one of clinical trials of statins, coronary events were suppressed by pravastatin treatment despite almost no changes in the degree of coronary stenosis evaluated with quantitative coronary angiography. This study indicated that statins prevent coronary events by some function other than the suppression of the degree of coronary stenosis. At that time, acute coronary syndromes are thought to occur with sudden coronary occlusion after rupture of coronary vulnerable lesions27–29). Therefore, statins' protective effects on coronary events were considered to be statin's atheroma stabilizing effects that prevent rupture of coronary lesions. Using mature WHHL-CA rabbits or WHHLMI rabbits that established coronary lesions, atheroma stabilizing effects of statins were examined (Fig. 1)30–33). In a quantitative analysis of lesion components34), compared to the placebo group, statin treatment increased collagen fibers and suppressed a decrease in smooth muscle cells during lesion progression, but treatment decreased macrophage derived foam cells and extracellular lipid accumulation. Consequently, an increase in lesion vulnerability score calculated by dividing the sum of the areas of macrophages and extracellular lipid by the sum of the areas of smooth muscle cells and collagen fibers was decreased. These observations about stabilization of coronary lesions were associated with a decrease in the expression of matrix metalloproteinases in atheromatous lesions31–33). In addition, studies using WHHL rabbits revealed that statin preserved the endothelium-dependent relaxation of the coronary arteries35) and improved disturbed endothelial barrier function36). Similar findings were observed in normal rabbits fed with a mild fat chow37–39). Recently, researchers reported that administration of statins after radiation therapy for cancer suppressed the onset of cerebrovascular and cardiovascular diseases40). These results in statin treatments may be related to statins' vascular endothelial cell protective effects and anti-inflammatory effects. Therefore, statins' anti-atherosclerotic effects are considered to be due mainly to lowering of plasma LDL cholesterol and protection of arterial endothelial cells. As described above, the WHHL rabbit family contributed significantly to the development and efficacy evaluation of statins.
Fig. 1.
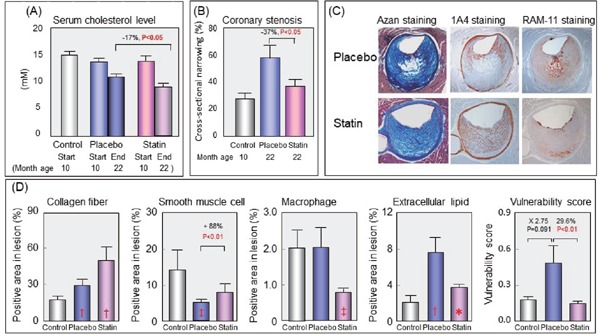
Suppressive effect of pravastatin on destabilization of coronary lesions in WHHL-CA rabbits
Coronary lesions were examined at the start of treatment (10 months old) in control group and at the end of treatment (22 months old) in placebo and statin groups. (A) Plasma cholesterol levels of each group at the start (10 months old) and the end of the treatment (22 months old), (B) The degree of coronary lesions of each group, (C) Photomicrographs of coronary lesions of placebo and pravastatin treated rabbits, and (D) Lesional composition of coronary lesions of each group. Vulnerability score was calculated by dividing sum of macrophage area and extracellular lipid area by sum of collagen area and smooth muscle cell area. Panels A–D are modified from Shiomi M, et al.30), and panel C is modified from Shiomi M, et al.31) *, P < 0.05; ‡, P < 0.01; †, P < 0.001 (vs. 10 months old control)
Development or Validation of the Lipid-Lowering Effects of Various Non-Statin Compounds Using the WHHL Rabbit Family
Lipid-lowering effects of various non-statin agents or molecules were examined in the WHHL rabbit family (Table 1). Similar to statins, various compounds and molecules showed lipid-lowering effects in the WHHL rabbit family. Since the microsomal triglyceride transfer protein (MTP) plays an important role in the assembly of VLDL particles in the liver and of chylomicron particles in the intestine41), MTP inhibitors lower plasma cholesterol levels by a mechanism different from statins. Lipid-lowering effects of MTP inhibitors were potent in WHHL-CA rabbits42), but a concern existed about lipid accumulation in the liver. Since a compound D-47, which exhibits unique lipid metabolism improving effects, decreased not only serum lipid levels but lipid accumulation in the liver and adipose tissues in WHHLMI rabbits43), D-47 may be effective to suppress lipid accumulation in the liver of animals treated with an MTP inhibitor. In addition, D-47 increased in the expression of CYP7A1, mRNA of cholesterol-7α-hydroxylase, and CPT-1, mRNA of carnitine palmitoyltransferase-1, but suppressed the expression of MTP and FAS, mRNA of fatty acid synthase, in the liver, and an increase in the expression of CPT-1 and LPL, mRNA of lipoprotein lipase, in the mesentery43). Therefore, D-47 may exhibit functions to prevent non-alcoholic fatty liver disease and metabolic syndrome. Further studies on D-47 are required. Triglyceride lowering effects in the WHHL rabbit family were observed with squalene synthase inhibitors44), MTP inhibitors42), fish oil45) or omega-3 fatty acids46), monocyte-specific colony-stimulating factor (M-CSF)47), granulocyte-macrophage colony-stimulating factor (GM-CSF)48), compound D-4743) but not with fibrates43, 49). Omega-3-fatty acids inhibit the assembly and secretion of VLDL particles50) and enhanced fatty acid oxidation in the liver51). Conversely, fibrates function through activation of the peroxisome proliferator-activated receptor alpha52). Since fenofibrate decreased plasma triglyceride levels in normal New Zealand white rabbits fed normal chow53), fibrates may not work well in hypercholesterolemia due to LDL receptor deficiency. Although the mechanism of the cholesterol-lowering effects of colony stimulating factors was not elucidated sufficiently, an increased clearance of apoB-100 containing lipoproteins through both LDL receptor-dependent and -independent pathways47) and an enhancement of VLDL receptor-mediated uptake of plasma VLDL48) may be involved. As described above, the WHHL rabbit family have been used to examine the lipid-lowering effects of various compounds, foods, and proteins.
Validation of the Anti-Atherosclerotic Effects of Various Non-Statin Compounds Using the WHHL Rabbit Family
Anti-atherosclerotic effects of various non-statin agents or molecules were examined in the WHHL rabbit family (Table 1). Similar to statins, a squalene synthase inhibitor, which inhibits cholesterol synthesis at a late stage in cholesterol biosynthesis pathway, also exhibits cholesterol-lowering effects and suppressed the destabilization and progression of atherosclerosis in WHHLMI rabbits44). Since squalene synthase inhibitors function distal to farnesyl pyrophosphate associated with the pleiotropic effects of statins54) and an increase in derivatives derived from farnesyl pyrophosphate is said to be associated with atherogenesis, the anti-atherosclerotic effects of squalene synthase inhibitors were considered to be due mainly to cholesterol- lowering effects. Other interesting molecules are M-CSF55) and GM-CSF56). M-CSF and GM-CSF are released activated cells in the arterial wall and can activate monocytes and macrophages57). However, M-CSF and GM-CSF suppressed atherosclerosis in WHHL-CA rabbits. Yamada et al.58) observed a decrease in the cellular cholesterol ester content in macrophages incubated with acetyl-LDL by the addition of M-CSF in the medium and an increase in plasma high-density lipoprotein (HDL)-cholesterol levels in WHHL-CA rabbits after M-CSF administration. These results suggest that atherosclerosis can be suppressed by normally activated macrophages or normalization of macrophage function. Regarding the anti-atherosclerotic effects of antihypertensive agents on normotensive WHHLMI rabbits, inhibitors of angiotensin converting enzyme59) and angiotensin-II type I receptor antagonists60) showed anti-atherosclerotic effects, but not calcium antagonists and beta-blockers. Angiotensin II promotes atherosclerosis through enhancement of cell proliferation, oxidative stress, suppression of arterial endothelial cell function, and inflammation through angiotensin II type I receptors in the arterial wall61). It is well known that an important risk factor for atherosclerosis is oxidized LDL. Anti-atherosclerotic effects of vitamins and compounds exhibiting anti-oxidative action have also been studied in WHHL-CA rabbits. Among various compounds and foods (vitamin E, alpha-tocopherol, herbal mixture, wine, and others) demonstrating anti-oxidative function, only probucol showed a clear anti-atherosclerosis effect in the WHHL rabbit family62–64). Although a case of QT-interval prolongation by probucol is a problem in humans with a missense mutation in the N-terminus of HERG gene65), QT-interval prolongation has not been observed in rabbits. As described above, the WHHL rabbit family have been used to examine the anti-atherosclerotic effects of various compounds, foods, and proteins.
Contribution of the WHHL Rabbit Family to the Development of Imaging Technologies for Atherosclerosis
The development of evaluation technologies for atherosclerotic lesions is of great importance in the diagnosis of atherosclerotic lesions and the evaluation of the therapeutic effects. Studies on atherosclerosis imaging are actively conducted using the WHHL rabbit family, and 71 papers have been reported. For imaging of vulnerable atheromatous lesions, several apparatuses have been used, such as positron emission tomography (PET), optical coherence tomography, computed tomography (CT), single photon emission computed tomography, magnetic resonance, intravascular ultrasound (IVUS), and others. In fact, these imaging technologies (CT-PET64), IVAS66), and iMAP IVUS67)) were effective in evaluating the therapeutic effects on atherosclerotic lesions. It is desirable that the animals used for imaging atherosclerosis demonstrate histopathologically similar arterial lesions as human lesions and are of a physical size that can be repeatedly imaged. The WHHL rabbit family was suitable for this condition.
The WHHL Rabbit Family as a Model of Gene Therapy
WHHL rabbits were also used in studies of gene therapy. Target genes were LDL receptor68, 69), apoB-editing enzyme70), monocyte chemoattractant protein- 1 (MCP-1)71), vascular endothelial growth factor (VEGF)72, 73), VEGF receptor74), endothelial nitric oxide synthase75), and β-galactosidase76). All of these gene therapies were effective in WHHL rabbits. Transfection of LDLR gene into WHHL liver using a lentiviral vector decreased serum cholesterol levels by 20–40% for one year without any side effects69). Transfection of a VEGF gene successfully mediated angiogenesis in skeletal muscle of WHHL rabbits72, 73). Thus, the WHHL rabbit family has also been used in studies of gene therapy, but further studies are needed to proceed gene therapy.
Translation of the Results of Animal Experiments into Humans
Although the WHHL rabbit family contributed to the development of therapeutics as described above, the Wall Street Journal issued February 24, 2004 said that the number of newly developed drugs decreased by approximately 60% in the United States from 1996 to 2003, despite the fact that the budget for new drug development increased almost doubled in this period. Since 2004, researchers frequently pointed out that an increasing discrepancy between the results of animal experiments and the results of clinical studies in the development of new drugs exists77–82). The possible causes are considered to be due to inadequate research target79, 80), insufficient study design77, 79–81), and species differences77–80) (Supplementary Table 1). The differences between the disease state of human patients and therapeutic conditions of animal experiments are summarized in Supplementary Table 2 77, 79). For reduction of the discrepancy between the results of animal experiments and human clinical studies, designing animal experiments that correspond to the pathophysiology of human patients is necessary, in addition to an adequate study target. Table 2 proposes considerations in the design of animal experiments for translation of the results of studies using animals into humans. In studies using animals (especially mice and rats), considering the effects of age, circadian rhythm, genetic homogeneity, gender, cause of disease, time to begin treatment, and drug doses is better. These differences between human patients and animals used in studies can affect the translation of the results of animal experiments into humans. Van der Worp et al.77) claimed that in animal studies the drug is administered at high doses that are toxic or intolerable to humans. Indeed, the dose of pravastatin to decrease serum cholesterol levels by approximately 30% without any side effects was 40 mg/body/day in humans83), 50 mg/kg/day in cynomolgus monkeys11), 3 mg/kg/day in beagle dogs84), 12.5 mg/kg/day in normal rabbits11), and 50 mg/kg/day in WHHL rabbits11). The causes of this species difference may be the difference in absorption of compounds in the intestine, degradation of the compounds in the liver, and the removal rate of the active form(s) of the compounds from the circulation. These results indicate that there are species differences in the effective dose of drugs, and the safety of the dose of drug that was effective in an efficacy test using animals should be examined in a safety study performed independently. The sample size in animal experiments is required to be as small as possible, from the viewpoint of animal welfare77, 81). However, studies with too few animals can lead to incorrect results. Calculating the number of animals needed for the experiment based on statistics is important.
Supplementary Table 1. Causes of poor reproducibility of the results of animal experiments in clinical trials.
Supplementary Table 2. Differences in condition between human patients and animals used in preclinical studies.
| Humans | Animals | |
|---|---|---|
| Age | Elder | Young |
| Presence of other diseases | Having other disease(s) | None |
| Circadian rhythm | Diurnality | Nocturnal |
| Genetic homogeneity | Individual differences | Homogenous strain |
| Gender | Both men and women | Males or females |
| Cause of disease | Spontaneous or disturbance of the immune or metabolism | Artificial induction or insufficient model |
| Time to begin treatment | After symptoms progress | At the time of induction or early stage |
| Drug dose | Extremely high dose |
Table 2. Considerations in the designing animal experiments.
|
Regarding species differences, lipoprotein metabolism, atherosclerosis, and myocardial function of mice and rats including genetically modified animals, these characteristics are very different from humans, but those of rabbits resemble human features (Supplementary Table 3)85–88). Fig. 2 shows lipoprotein profiles and plasma lipid levels of healthy humans and several animal models. In human familial hypercholesterolemia homozygotes who are deficient in LDL receptors, the serum cholesterol levels are more than 500 mg/dl due to an elevation of LDL cholesterol levels. The WHHL family also showed a marked elevation of LDL fraction (Fig. 2), due to LDL receptor deficiency. However, in LDLR-KO mice fed standard chow, the serum cholesterol levels were 218 ± 17 mg/dl, and the elevation of LDL cholesterol levels was mild (Fig. 2)89). Similar findings were observed in LDLR-KO rats90). Since overexpression of apoB-100 in LDLR-KO mice liver markedly increased LDL-cholesterol levels 91) and LDL-cholesterol levels decreased markedly in WHHL rabbits expressed apoB mRNA editing enzyme (APOBECK-1) in the livers70), these differences between human patients or the WHHL family and mice or rats may be due to expression of APOBECK-1 in the liver of mice and rats. Lipoprotein profiles of apoE-KO mice, another mice model for hypercholesterolemia, are also very different from human hypercholesterolemia (Fig. 2). In apoE-KO mice, the VLDL fraction is increased markedly, but the VLDL particles contain apoB-48 instead of apoB-100 92). In addition, no cholesterol ester-transfer protein activity in the plasma93), high activity of hepatic LDL receptor function86), and high activity of hepatic lipase in pre-heparin plasma94) in mice are also very different from humans. However, those of rabbits are close to humans85–88). In particular, the cholesterol-lowering effects of statins were hardly observed in mice95) and rats9, 11, 96) but were potent in rabbits and WHHL rabbit family11–20, 23, 30–34). In rats treated with statins, the activities of hepatic HMG-CoA reductase96) and synthesis of hepatic fatty acids96) were increased markedly. In mice, high activity of hepatic LDL receptor, high excretion of bile acid, and secretion of VLDL with apoB-48 from liver88) may be associated with the lack of the effects of statins. Conversely, fibrates are effective in rats and normal rabbits but not in the WHHL rabbit family. Recently, monoclonal antibodies against proprotein convertase subtilisin/kexin type 9 (PCSK9) that inhibit lysosomal degradation of LDL receptor proteins have been developed, and mice have also been used for the development of PCSK9 antibodies97). Also, with respect to atherosclerosis and the characteristics of myocardium, mouse models are different from humans, but rabbits are similar to humans85–88). In humans and WHHLMI rabbits, macrophages in atherosclerotic lesions express VLDL receptors but not in LDLR-KO mice and apoE-KO mice98). These findings suggest that the mechanism of atherogenesis in mouse models may be different from those of humans and WHHLMI rabbits. Finally, Fan et al.85) reported that distinctive phenotypes of lipoprotein metabolism and the onset of atherosclerosis differ between mice and rabbits that have been transfected the same gene. These observations about species differences give us the following two lessons: 1) In order to extrapolate the results of animal experiments to humans, using appropriate animal models in which pathogenesis and enzymes in the related metabolic pathway closely match humans is necessary, in addition to serological data such as total cholesterol levels; 2) Not all genetically modified animals correspond to human diseases. From these viewpoints, the WHHL rabbit family are suitable animal models for studies of lipid metabolism, atherosclerosis, and the development of the therapeutics. The National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) proposed ARRIVE Guidelines to improve the discrepancy between the results of animal experiments and clinical research99). In order to translate the results of studies using animals to humans, in addition to appropriate study designs and appropriate treatment targets, using animals corresponding to human conditions is important (Table 3). To perform translatable animal experiments, researchers should consider study design and animals used in studies more carefully.
Supplementary Table 3. Species differences in lipoprotein metabolism, atherosclerosis, and myocardial characteristics.
| Humans | WHHLMI rabbits | Mice | |
|---|---|---|---|
| Lipoprotein metabolism | |||
| Main lipoprotein | LDL | LDL | HDL, VLDL with apoB48 |
| ApoB of VLDL | ApoB-100 | ApoB-100 | apoB-48 |
| Expression of apoB editing enzyme | ilium | ilium | ilium and liver |
| CETP in plasma | Yes | Yes | none |
| Hepatic lipase activity in pre-heparin plasma | very low | very low | high |
| Response to dietary lipid | sensitive | sensitive | resistant |
| Effects of endothelial lipase on LDL | no effects | no effects | lowering of LDL |
| Cholesterol-lowering effects of statin | effective | effective | ineffective |
| Apolipoprotein(a) | Bound to LDL | Bound to LDL | Not bound to LDL |
| HDL | heterogeneous | heterogeneous | homogenous |
| Apolipoprotein AII | Dimmer | Absent | Monomer |
| Hepatic LDL receptor activity | Down regulated | Down regulated | Usually high |
| Main cholesterol pool | hepatic synthesis | hepatic synthesis | dietary origin |
| Excretion of bile acid | Low | Low | High |
| Atherosclerosis | |||
| Atherogenesis | Sensitive | Spontaneous | Resistant |
| Coronary lesions | Frequent | Frequent | Rare |
| Feature of coronary lesion | Various types | Various types | Excessive lipid deposition |
| Expression of VLDL receptors in lesions | Macrophages | Macrophages | no expression |
| Destabilization of plaques by MMPs | Yes | Yes | Inconsistent results |
| Acute inflammation marker | CRP | CRP | SAP |
| Cardiac function | |||
| Cardiac myosin | |||
| Myosin heavy chain | β-type | β-type | α-type |
| Ion channel of myocardial myosin | Ikr and Iks | Ikr and Iks | Ito and Ik, slow |
| ECG | 12-lead ECG | 12-lead ECG | Single lead ECG |
| Wave form of ECG | T-wave | T-wave | J-wave |
Fig. 2.
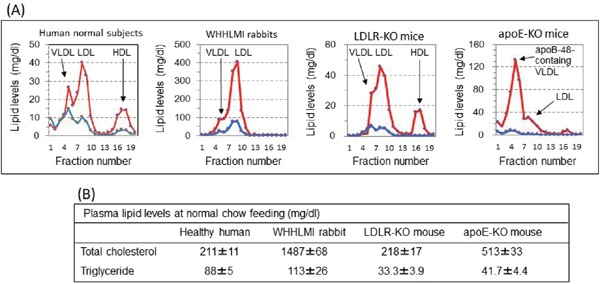
Lipoprotein profiles (A) and plasma lipid levels (B) of healthy humans, WHHLMI rabbits, LDLR-KO mice, and apoE-KO mice
Lipoprotein profiles were analyzed with high performance liquid chromatography87). Animals were fed standard chow. Red lines indicate cholesterol and blue lines indicate triglyceride.
Table 3. Conditions of animals used in studies to translate the results into humans.
|
Conclusion
With the end of breeding of the WHHL rabbit family at Kobe University, this review summarized the contribution of the WHHL rabbit family to the development of therapeutics for hypercholesterolemia and atherosclerosis. Studies using the WHHL rabbit family demonstrated that using animals suitable for study purpose is important for translating the results into humans. Information on the WHHL rabbit family and a list of studies using the WHHL rabbit family that can be used for subject searches can be found on the WHHL website (http://www.med.akita-u.ac.jp/∼doubutu/WHHL/WHHL-home.html).
Acknowledgments
This author is grateful to the many pharmaceutical companies listed below for supporting keeping the WHHL rabbit family; Sankyo Co., Ltd, Japan; Daiichi-Sankyo Co., Ltd, Japan; Takeda Pharmaceutical Company, Japan; Nippon Shinyaku Co. Ltd., Japan; Bayer Yakuhin, Ltd., Japan; Novartis Pharma K.K., Japan; Otsuka Pharmaceutical Co. Ltd., Japan; Banyu Pharmaceutical Co., Ltd., Japan, Shionogi & Co., Ltd, Japan; Taisho Pharmaceutical Co., Ltd., Japan; Kissei Pharmaceutical Co., Ltd., Japan; Ono Pharmaceutical Co., Ltd., Japan; Fujirebio Inc., Japan; Asahi Kasei Pharmaceutical Co., Ltd., Japan; Mitsubishi Tanabe Pharma Co., Ltd., Japan; and Warner Lambert Japan Co., Ltd., Japan. This author is grateful to the researchers who used WHHL rabbit family effectively for their studies, and expresses sincere appreciation for the continuous efforts of the staff of the Institute for Experimental Animals, Kobe University Graduate School of Medicine (Kobe, Japan) in maintaining the WHHL rabbit family.
Author contribution
MS prepared this manuscript.
Conflicts of Interests
There is no conflict of interest associated with this manuscript within the past 36 months.
References
- 1). Watanabe Y: Serial inbreeding of rabbits with hereditary hyperlipidemia (WHHL-rabbit). Atherosclerosis, 1980; 36: 261-268 [DOI] [PubMed] [Google Scholar]
- 2). Tanzawa K, Shimada Y, Kuroda M, Tsujita Y, Arai M, Watanabe Y: WHHL-rabbit: A low density lipoprotein receptor-deficient animal model for familial hypercholesterolemia. FEBS Lett, 1980; 118: 81-84 [DOI] [PubMed] [Google Scholar]
- 3). Kita T, Brown MS, Watanabe Y, Goldstein JL: Deficiency of low density lipoprotein receptors in liver and adrenal gland of the WHHL rabbit, an animal model of familial hypercholesterolemia. Proc Natl Acad Sci USA, 1981; 78: 2268-2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Watanabe Y, Ito T, Shiomi M: The effect of selective breeding on the development of coronary atherosclerosis in WHHL rabbits, an animal model for familial hypercholesterolemia. Atherosclerosis, 1985; 56: 71-79 [DOI] [PubMed] [Google Scholar]
- 5). Shiomi M, Ito T, Shiraishi M, Watanabe Y: Inheritability of atherosclerosis and the role of lipoproteins as risk factors in the development of atherosclerosis in WHHL rabbits: Risk factors related to coronary atherosclerosis are different from those related to aortic atherosclerosis. Atherosclerosis, 1992; 96: 43-52 [DOI] [PubMed] [Google Scholar]
- 6). Shiomi M, Ito T, Yamada S, Kawashima S, Fan J: Development of an animal model for spontaneous myocardial infarction (WHHLMI rabbit). Arterioscler Thromb Vasc Biol, 2003; 23: 1239-1244 [DOI] [PubMed] [Google Scholar]
- 7). Shiomi M: The history of the WHHL rabbit, an animal model of familial hypercholesterolemia (I) - Contribution to elucidation of the pathophysiology of human hypercholesterolemia and coronary heart disease -. J Atheroscler Thromb (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Endo A, Kuroda M, Tsujita Y: ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium. J Antibiot (Tokyo), 1976; 29: 1346-1348 [DOI] [PubMed] [Google Scholar]
- 9). Endo A: The discovery and development of HMG-CoA reductase inhibitors. J Lipid Re, 1992; 33: 1569-1582 [PubMed] [Google Scholar]
- 10). Watanabe Y, Ito T, Saeki M, Kuroda M, Tanzawa K, Mochizuki M, Tsujita Y, Arai M: Hypolipidemic effects of CS-500 (ML-236B) in WHHL-rabbit, a heritable animal model for hyperlipidemia. Atherosclerosis, 1981; 38: 27-31 [DOI] [PubMed] [Google Scholar]
- 11). Tsujita Y, Kuroda M, Shimada Y, Tanzawa K, Arai M, Kaneko I, Tanaka M, Masuda H, Tarumi C, Watanabe Y, Fujii S: CS-514, a competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase: Tissue-selective inhibition of sterol synthesis and hypolipidemic effect on various animal species. Biochim Biophys Acta, 1986; 877: 50-60 [DOI] [PubMed] [Google Scholar]
- 12). Ma PTS, Gil G, Sudhof TC, Billheimer DW, Goldstein JL, Brown MS: Mevinolin, an inhibitor of cholesterol synthesis, induces mRNA for low density lipoprotein receptor in livers of hamsters and rabbits. Proc Natl Acad Sci USA, 1986; 83: 8370-8374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Shiomi M, Yamada S, Ito T: Atheroma stabilizing effects of simvastatin due to depression of macrophages or lipid accumulation in the atheromatous plaques of coronary atherosclerosis-prone WHHL rabbits. Atherosclerosis, 2005; 178: 287-294 [DOI] [PubMed] [Google Scholar]
- 14). Shiomi M, Shiraishi M, Yata T, Ito T: Effect of fluvastatin sodium on secretion of very low density lipoprotein and serum cholesterol levels: In vivo study using low density lipoprotein receptor deficient Watanabe heritable hyperlipidemic rabbits. Arzneim Forsch/Drug Res, 1994; 44: 1154-1156 [PubMed] [Google Scholar]
- 15). Shiomi M, Ito T: Effects of cerivastatin sodium, a new inhibitor of HMG-CoA reductase, on plasma lipid levels, progression of atherosclerosis, and the lesional composition in the plaques of WHHL rabbits. Br J Pharmacol, 1999; 126: 961-968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Suzuki H, Kobayashi H, Sato F, Yonemitsu Y, Nakashima Y, Sueishi K: Plaque-stabilizing effect of pitavastatin in Watanabe heritable hyperlipidemic (WHHL) rabbits. J Atheroscler Thromb, 2003; 10: 109-116 [DOI] [PubMed] [Google Scholar]
- 17). Li S, Liang J, Niimi M, Waqar AB, Kang D, Koike T, Wang Y, Shiomi M, Fan J: Probucol Suppresses Macrophage Infiltration and MMP Expression in Atherosclerotic Plaques of WHHL Rabbits. J Atheroscler Thromb, 2014; 21: 648-658 [DOI] [PubMed] [Google Scholar]
- 18). Kuroda M, Matsumoto A, Itakura H, Watanabe Y, Ito T, Shiomi M, Fukushige J, Nara F, Fukami M, Tsujita Y: Effects of pravastatin sodium alone and in combination with cholestyramine on hepatic, intestinal and adrenal low density lipoprotein receptors in homozygous Watanabe heritable hyperlipidemic rabbits. Japan J Phamacol, 1992; 59: 65-70 [DOI] [PubMed] [Google Scholar]
- 19). Kurokawa J, Hayashi K, Toyota Y, Shingu T, Shiomi M, Kajiyama G: High dose of fluvastatin sodium (Xu62- 320), a new inhibitor of 3-hydroxy-3-merhylglutaryl coenzyme A reductase, lowers plasma cholesterol levels in homozygous Watanabe-heritable hyperlipidemic rabbits. Biochim Biophys Acta, 1995; 1259: 99-104 [DOI] [PubMed] [Google Scholar]
- 20). Schneider WJ, Brown MS, Goldstein JL: Kinetic defects in the processing of the low density lipoprotein receptor in fibroblasts from WHHL rabbits and a family with familial hypercholesterolemia. Mol Biol Med, 1983; 1: 353-367 [PubMed] [Google Scholar]
- 21). Shiomi M, Ito T, Watanabe Y: Increase in hepatic low-density lipoprotein receptor activity during pregnancy in Watanabe heritable hyperlipidemic rabbits, an animal model for familial hypercholesterolemia. Biochim Biophys Acta, 1987; 917: 92-100 [DOI] [PubMed] [Google Scholar]
- 22). Mabuchi H, Sakai Y, Watanabe A, Haba T, Koizumi J, Takeda R: Normalization of low-density lipoprotein levels and disappearance of xanthomas during pregnancy in a woman with heterozygous familial hypercholesterolemia. Metabolism, 1985; 34: 309-315 [DOI] [PubMed] [Google Scholar]
- 23). Shiomi M, Ito T: Pravastatin sodium, a competitive inhibitor of hepatic 3-hydoroxy-3-methylglutaryl coenzyme A reductase, decreases the cholesterol content of newly secreted very-low-density lipoprotein in Watanabe heritable hyperlipidemic rabbits. Metabolism, 1994; 43: 559-564 [DOI] [PubMed] [Google Scholar]
- 24). Watanabe Y, Ito T, Shiomi M, Tsujita Y, Kuroda M, Arai M, Fukami M, Tamura A: Preventive effect of pravastatin sodium, a potent inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase, on coronary atherosclerosis and xanthoma in WHHL rabbits. Biochim Biophys Acta, 1988; 960: 294-302 [PubMed] [Google Scholar]
- 25). Shiomi M, Ito T, Watanabe Y, Tsujita Y, Kuroda M, Arai M, Fukami M, Fukushige J, Tamura A: Suppression of established atherosclerosis and xanthomas in mature WHHL rabbits by keeping their serum cholesterol levels extremely low: Effect of pravastatin sodium in combination with cholestyramine. Atherosclerosis, 1990; 83: 69-80 [DOI] [PubMed] [Google Scholar]
- 26). Jukema JW, Bruschke AVG, van Boven AJ, Reiber JHC, Bal ET, Zwinderman AH, Jansen H, Boerma GJM, van Rappard FM, Lie KI: Effects of lipid lowering by pravastatin on progression and regression of coronary artery disease in symptomatic men with normal to moderately elevated serum cholesterol levels. The Regression Growth Evaluation Statin Study (REGRESS). Circulation, 1995; 91: 2528-2540 [DOI] [PubMed] [Google Scholar]
- 27). van der Wal AC, Becker AE, van der Loos CM, Das PK: Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation, 1994; 89: 36-44 [DOI] [PubMed] [Google Scholar]
- 28). Fuster V, Badimon L, Badimon JJ, Chesebro JH: The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N Engl J Med, 1992; 326: 242-250 [DOI] [PubMed] [Google Scholar]
- 29). Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Juhani Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W, Jr, Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT: From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation, 2003; 108: 1664-1672 [DOI] [PubMed] [Google Scholar]
- 30). Shiomi M, Ito T, Tsukada T, Yata T, Watanabe Y, Tsujita Y, Fukami M, Fukushige J, Hosohawa T, Tamura A: Reduction of serum cholesterol levels alters lesional composition of atherosclerotic plaques: Effect of pravastatin sodium on atherosclerosis in mature WHHL rabbits. Arterioscler Thromb Vasc Biol, 1995; 15 1938-1944 [DOI] [PubMed] [Google Scholar]
- 31). Shiomi M, Ito T, Hirouchi Y, Enomoto M: Fibromuscular cap composition is important for the stability of established atherosclerotic plaques in mature WHHL rabbits treated with statins. Atherosclerosis, 2001; 157: 75-84 [DOI] [PubMed] [Google Scholar]
- 32). Fukumoto Y, Libby P, Rabkin E, Hill CC, Enomoto M, Hirouchi Y, Shiomi M, Aikawa M: Statins alter smooth muscle cell accumulation and collagen content in established atheroma of Watanabe heritable hyperlipidemic rabbits. Circulation, 2001; 103: 993-999 [DOI] [PubMed] [Google Scholar]
- 33). Shiomi M, Yamada S, Ito T: Atheroma stabilizing effects of simvastatin due to depression of macrophages or lipid accumulation in the atheromatous plaques of coronary atherosclerosis-prone WHHL rabbits. Atherosclerosis, 2005; 178: 287-294 [DOI] [PubMed] [Google Scholar]
- 34). Shiomi M, Ito T, Tsukada T, Yata T, Ueda M: Cell compositions of coronary and aortic atherosclerotic lesions in WHHL rabbits differ: an immunohistochemical study. Arterioscler Thromb, 1994; 14: 931-937 [DOI] [PubMed] [Google Scholar]
- 35). Kroon AA, Stalenhoef AFH, Buikema H, Demacker PNM, de Wilde PCM, Leijten PA, van Gilst WH: The effect of cholesterol reduction on the endothelial function and progression of atherosclerosis in WHHL rabbits. Atherosclerosis, 1993; 103: 221-230 [DOI] [PubMed] [Google Scholar]
- 36). Amerongen GPV, Vermeer MA, Negre-Aminou P, Lankelma J, Emeis JJ, van Hinsbergh VWM: Simvastatin improves disturbed endothelial barrier function. Circulation, 2000; 102: 2803-2809 [DOI] [PubMed] [Google Scholar]
- 37). Aikawa M, Sugiyama S, Hill CC, Voglic SJ, Rabkin E, Fukumoto Y, Schoen FJ, Witztum JL, Libby P: Lipid lowering reduces oxidative stress and endothelial cell activation in rabbit atheroma. Circulation, 2002; 106: 1390-1396 [DOI] [PubMed] [Google Scholar]
- 38). Aikawa M, Rabkin E, Okada Y, Voglic SJ, Clinton SK, Brinckerhoff CE, Sukhova GK, Libby P: Lipid lowering by diet reduces matrix metalloproteinase activity and increases collagen content of rabbit atheroma: a potential mechanism of lesion stabilization. Circulation, 1998; 97: 2433-24244 [DOI] [PubMed] [Google Scholar]
- 39). Aikawa M, Voglic SJ, Sugiyama S, Rabkin E, Taubman MB, Fallon JT, Libby P: Dietary lipid lowering reduces tissue factor expression in rabbit atheroma. Circulation, 1999; 100: 1215-1222 [DOI] [PubMed] [Google Scholar]
- 40). Boulet J, Pena J, Hulten EA, Neilan TG, Dragomir A, Freeman C, Lambert C, Hijal T, Nadeau L, Brophy JM, Mousavi N: Statin Use and Risk of Vascular Events Among Cancer Patients After Radiotherapy to the Thorax, Head, and Neck. J Am Heart Assoc, 2019; 8: e005996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Hussain MM, Shi J, Dreizen P: Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J Lipid Res, 2003; 44: 22-32 [DOI] [PubMed] [Google Scholar]
- 42). Shiomi M, Ito T: MTP inhibitor decreases plasma cholesterol levels in LDL receptor-deficient WHHL rabbits by lowering the VLDL secretion. Eur J Pharmacol, 2001; 431: 127-131 [DOI] [PubMed] [Google Scholar]
- 43). Tamura S, Koike Y, Takeda H, Koike T, Izumi Y, Nagasaka R, Tsunoda T, Tori M, Ogawa K, Bamba T, Shiomi M: Ameliorating effects of D-47, a newly developed compound, on lipid metabolism in an animal model of familial hypercholesterolemia (WHHLMI rabbits). Eur J Pharmacol, 2018; 822: 147-153 [DOI] [PubMed] [Google Scholar]
- 44). Shiomi M, Yamada S, Amano Y, Nishimoto T, Ito T: Lapaquistat acetate, a squalene synthesis inhibitor, changes macrophage/lipid-rich coronary plaques of hypercholesterolemic rabbits into fibrous lesions. Br J Pharmacol, 2008; 154: 949-957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45). Rich S, Miller JM, Charous S, Davis HR, Shanks P, Glagov S, Lands WEM: Development of atherosclerosis in genetically hyperlipidemic rabbits during chronic fish-oil ingestion. Arteriosclerosis, 1989; 9: 189-194 [DOI] [PubMed] [Google Scholar]
- 46). Clubb FJ, Schmitz JM, Butler MM, Buja LM, Willerson JT, Campbell WB: Effect of dietary omega-3 fatty acid on serum lipids, platelet function, and atherosclerosis in Watanabe heritable hyperlipidemic rabbits. Arteriosclerosis, 1989; 9: 529-537 [DOI] [PubMed] [Google Scholar]
- 47). Shimano H, Yamada N, Ishibashi S, Harada K, Matsumoto A, Mori N, Inaba T, Motoyoshi K, Itakura H, Takaku F: Human monocyte colony-stimulating factor enhances the clearance of lipoproteins containing apolipoprotein B-100 via both low density lipoprotein receptor-dependent and-independent pathways in rabbits. J Biol Chem, 1990; 265: 12869-12875 [PubMed] [Google Scholar]
- 48). Ishibashi T, Yokoyama K, Shindo J, Hamazaki Y, Endo Y, Sato T, Takahashi S, Kawabayashi Y, Shiomi M, Yamamoto T, Maruyama Y: Potent cholesterol-lowering effect by human granulocyte-macrophage colony-stimulating factor in rabbits: possible implications of enhancement of macrophage function and an increase in mRNA for VLDL receptor. Arterioscler Thromb, 1994; 14: 1534-1541 [DOI] [PubMed] [Google Scholar]
- 49). Liu R, Saku K, Jimi S, Ohta T, Zhang B, Takebayashi S, Arakawa K: Mechanism of action of gemfibrozil on HDL metabolism and atherosclerosis in WHHL rabbits. Cardiovasc Drug Therapy, 1997; 11: 659-668 [DOI] [PubMed] [Google Scholar]
- 50). Lang CA, Davis RA: Fish oil fatty acids impair VLDL assembly and/or secretion by cultured rat hepatocytes. J Lipid Res, 1990; 31: 2079-2086 [PubMed] [Google Scholar]
- 51). Berge RK, Madsen L, Vaagenes H, Tronstad KJ, Gottlicher M, Rustan AC: In contrast with docosahexaenoic acid, eicosapentaenoic acid and hypolipidaemic derivatives decrease hepatic synthesis and secretion of triacylglycerol by de-creased diacylglycerol acyltransferase activity and stimulation of fatty acid oxidation. Biochem J, 1999; 343: 191-197 [PMC free article] [PubMed] [Google Scholar]
- 52). Schoonjans K, Staels B, Auwerx J: Role of the peroxisome proliferator activated receptor (PPAR) in mediating effects of fibrates and fatty acids on gene expression. J Lipid Res, 1996; 37: 907-925 [PubMed] [Google Scholar]
- 53). Mondragón-García A, Luna-Luna M, Flores-Castillo C, Aranda-Fraustro A, Carreón-Torres E, López-Olmos V, Fragoso JM, Vargas-Alarcón G, Pérez-Méndez Ó: Atorvastatin and fenofibrate exert opposite effects on the vascularization and characteristics of visceral adipose tissue in New Zealand white rabbits. J Cardiovasc Pharmacol Ther, 2019: 1074248419838517. [DOI] [PubMed] [Google Scholar]
- 54). Ludman A, Venugopal V, Yellon DM, Hausenloy DJ: Statins and cardioprotection--more than just lipid lowering? Pharmacol Ther, 2009; 122: 30-43 [DOI] [PubMed] [Google Scholar]
- 55). Inoue I, Inaba T, Motoyoshi K, Harada K, Shimano H, Kawamura M, Gotoda T, Oka T, Shiomi M, Watanabe Y, Tsukada T, Yazaki Y, Takaku F, Yamada N: Macrophage colony stimulating factor prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbits. Atherosclerosis, 1992; 93(3); 245-254 [DOI] [PubMed] [Google Scholar]
- 56). Shindo J, Ishibashi T, Yokoyama K, Ohwada T, Shiomi M, Maruyama Y: Granulocyte-macrophage colony-stimulating factor prevents the progression of atherosclerosis via changes in the cellular and extracellular composition of atherosclerotic lesions in Watanabe heritable hyperlipidemic rabbits. Circulation, 1999; 99: 2150-2156 [DOI] [PubMed] [Google Scholar]
- 57). Filonzi EL, Zoellner H, Stanton H, Hamilton JA: Cytokine regulation of granulocyte-macrophage colony stimulating factor and macrophage colony-stimulating factor production in human arterial smooth muscle cells. Atherosclerosis, 1993; 99: 241-252 [DOI] [PubMed] [Google Scholar]
- 58). Yamada N, Ishibashi S, Shimano H, Inaba T, Gotoda T, Harada K, Shimada M, Shiomi M, Watanabe Y, Kawakami M, Yazaki Y, Takaku F: Role of monocyte colony-stimulating factor in form cell generation. Proc Soc Exp Biol Med, 1992; 200: 240-244 [DOI] [PubMed] [Google Scholar]
- 59). Chobanian AV, Haudenschild CC, Nickerson C, Drago R: Antiatherogenic effect of captopril in the Watanabe heritable hyperlipidemic rabbit. Hypertension, 1990; 15: 327-331 [DOI] [PubMed] [Google Scholar]
- 60). Imanishi T, Kuroi A, Ikejima H, Kobayashi K, Muragaki Y, Mochizuki S, Goto M, Yoshida K, Akasaka T: Effects of angiotensin converting enzyme inhibitor and angiotensin II receptor antagonist combination on nitric oxide bioavailability and atherosclerotic change in Watanabe heritable hyperlipidemic rabbits. Hypertens Res, 2008; 31: 575-584 [DOI] [PubMed] [Google Scholar]
- 61). Montezano AC, Nguyen Dinh, Cat A, Rios FJ, Touyz RM: Angiotensin II and vascular injury. Curr Hypertens Rep, 2014; 16: 431-442 [DOI] [PubMed] [Google Scholar]
- 62). Kita T, Nagano Y, Yokode M, Ishii K, Kume N, Ooshima A, Yoshida H, Kawai C: Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci USA, 1987; 84: 5928-5931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63). Carew TE, Schwenke DC, Steinberg D: Antiatherogenic effect of probucol unrelated to its hypercholesterolemic effect: Evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbits. Proc Natl Acad Sci USA, 1987; 84: 7725-7729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64). Ogawa M, Magata Y, Kato T, Hatano K, Ishino S, Mukai T, Shiomi M, Ito K, Saji H: Application of 18F-FDG PET for monitoring the therapeutic effect of antiinflammatory drugs on stabilization of vulnerable atherosclerotic plaques. J Nucl Med, 2006; 47: 1845-1850 [PubMed] [Google Scholar]
- 65). Hayashi K, Shimizu M, Ino H, Yamaguchi M, Terai H, Hoshi N, Higashida H, Terashima N, Uno Y, Kanaya H, Mabuchi H: Probucol aggravates long QT syndrome associated with a novel missense mutation M124T in the N-terminus of HERG. Clin Sci (Lond), 2004; 107: 175-182 [DOI] [PubMed] [Google Scholar]
- 66). Iwata A, Miura SI, Zhang B, Imaizumi S, Uehara Y, Shiomi M, Saku K: Antiatherogenic effects of newly developed apolipoprotein A-I mimetic peptide/phospholipid complexes against aortic plaque burden in Watanabe-heritable hyperlipidemic rabbits. Atherosclerosis, 2011; 218: 300-307 [DOI] [PubMed] [Google Scholar]
- 67). Sudo M, Li Y, Hiro T, Takayama T, Mitsumata M, Shiomi M, Sugitani M, Matsumoto T, Hao H, Hirayama A: Inhibition of plaque progression and promotion of plaque stability by glucagon-like peptide-1 receptor agonist: Serial in vivo findings from iMap-IVUS in Watanabe heritable hyperlipidemic rabbits. Atherosclerosis, 2017; 265: 283-291 [DOI] [PubMed] [Google Scholar]
- 68). Hytönen E, Laurema A, Kankkonen H, Miyanohara A, Karja V, Hujo M, Laham-Karam N, Yla-Herttuala S: Bileduct proliferation as an unexpected side-effect after AAV2-LDLR gene transfer to rabbit liver. Sci Rep, 2019. 6; 9: 6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69). Chowdhury JR, Grossman M, Gupta S, Chowdhury NR, Baker JR, Wilson JM: Long-term improvement of hypercholesterolemia after ex vivo gene therapy LDLR-deficient rabbits. Science, 1991; 254: 1802-1805 [DOI] [PubMed] [Google Scholar]
- 70). Greeve J, Jona VK, Chowdhury NR, Horwitz MS, Chowdhury JR: Hepatic gene transfer of the catalytic subunit of the apolipoprotein B mRNA editing enzyme results in a reduction of plasma LDL levels in normal and Watanabe heritable hyperlipidemic rabbits. J Lipid Res, 1996; 37: 2001-2017 [PubMed] [Google Scholar]
- 71). van Royen N, Hoefer I, Buschmann I, Kostin S, Voskuil M, Bode CH, Schaper W, Piek JJ: Effects of local MCP-1 protein therapy on the development of the collateral circulation and atherosclerosis in Watanabe hyperlipidemic rabbits. Cardiovasc Res, 2003; 57: 178-185 [DOI] [PubMed] [Google Scholar]
- 72). Shyu K, Chang H, Isner JM: Synergistic effect of angiopoietin-1 and vascular endothelial growth factor on neoangiogenesis in hypercholesterolemic rabbit model with acute hind limb ischemia. Life Sci, 2003; 73: 563-579 [DOI] [PubMed] [Google Scholar]
- 73). Roy H, Bhardwaj S, Babu M, Lahteenvuo JE, Yla-Hertuala S: VEGF-DΔNΔC mediated angiogenesis in skeletal muscles of diabetic WHHL rabbits. Eur J Clin Invest, 2010; 40: 422-432 [DOI] [PubMed] [Google Scholar]
- 74). Hytonen JP, Taavitsainen J, Laitinen JTT, Partanen A, Alitalo K, Leppanen O, Yla-Herttuala S: Local adventitial anti-angiogenic gene therapy reduces growth of vasa-vasorum and in-stent restenosis in WHHL rabbits. J Mol Cell Cardiol, 2018; 121: 145-154 [DOI] [PubMed] [Google Scholar]
- 75). Ooboshi H, Toyoda K, Faraci FM, Lang MG, Heistad DD: Improvement of relaxation in an atherosclerotic artery by gene transfer of endothelial nitric oxide synthase. Arterioscler Thromb Vasc Biol, 1998; 18: 1752-1758 [DOI] [PubMed] [Google Scholar]
- 76). Lund DD, Faraci FM, Ooboshi H, Davidson BL, Heistad DD: Adenovirus-mediated gene transfer is augmented in basilar and carotid arteries of heritable hyperlipidemic rabbits. Stroke, 1999; 30: 120-125 [DOI] [PubMed] [Google Scholar]
- 77). van der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, O'Collins V, Macleod MR: Can animal models of disease reliably inform human studies? PLoS Med, 2010; 7: e1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78). Wadman M: NIH mulls rules for validating key results. Nature, 2013; 500: 14-16 [DOI] [PubMed] [Google Scholar]
- 79). Couzin-Frankel J: When mice Mislead. Science, 2013; 342: 922-923 [DOI] [PubMed] [Google Scholar]
- 80). Denayer T, Stohr T, VanRoy M: Animal models in translational medicine: Validation and prediction. New Horiz Transl Med, 2014; 2: 5-11 [Google Scholar]
- 81). Nature Editorials : Numbers matter, Researchers need help in making the statistical power of animal experiments clear. Nature, 2015; 520: 263-264 [Google Scholar]
- 82). Ramirez FD, Motazedian P, Jung RG, Di Santo P, Mac-Donald ZD, Moreland R, Simard T, Clancy AA, Russo JJ, Welch VA, Wells GA, Hibbert B: Methodological Rigor in Preclinical Cardiovascular Studies: Targets to Enhance Reproducibility and Promote Research Translation. Cir Res, 2017; 120: 1916-1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83). McTavish D, Sorkin EM: Pravastatin-A review of its pharmacological properties and therapeutic potential in hypercholesterolemia. Drugs, 1991; 42: 65-89 [DOI] [PubMed] [Google Scholar]
- 84). Fujioka T, Nara F, Shimada Y, Fukushige J, Shimotsu H, Shigehara E, Fukami M, Tsujita Y: The mechanism of comparable serum cholesterol lowering effects of pravastatin sodium, a 3-hydroxy-3-methylglutaryl coenzyme A inhibitor, between once- and twice-daily treatment regimens in beagle dogs and rabbits. Jpn J Pharmacol, 1996; 70: 329-335 [DOI] [PubMed] [Google Scholar]
- 85). Fan J, Watanabe T: transgenic rabbits as therapeutic protein bioreactors and human disease models. Pharmacol Ther, 2003; 99: 261-282 [DOI] [PubMed] [Google Scholar]
- 86). Fan J, Chen Y, Yan H, Niimi M, Wang Y, Liang J: Principles and applications of rabbit models for atherosclerosis research. J Atheroscler Thromb, 2018; 25: 213-220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87). Shiomi M, Koike T, Ishida T: Genetically Modified Animal Models for Lipoprotein Research. In Frank S, Kostner G. eds. Lipoproteins - Role in Health and Diseases. InTechopen.com. (University Campus STeP Ri, Rijeka, Croatia), 2012, Chapter 22, pp 533-560 [Google Scholar]
- 88). Fan J, Kitajima S, Watanabe T, Xu J, Zhang J, Liu E, Chen YE: Rabbit models for the study of human atherosclerosis: From pathophysiological mechanisms to translational medicine. Pharmacol Ther, 2015; 146: 104-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89). Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J: Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest, 1993; 92: 883-893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90). Asahina M, Mashimo T, Takeyama M, Tozawa R, Hashimoto T, Takizawa A, Ueda M, Aoto T, Kuramoto T, Serikawa T: Hypercholesterolemia and atherosclerosis in low density lipoprotein receptor mutant rats. Biochem Biophys Res Commun, 2012; 418: 553-558 [DOI] [PubMed] [Google Scholar]
- 91). Sanan DA, Newland DL, Tao R, Marcovina S, Wang J, Mooser V, Hammer RE, Hobbs HH: Low density lipoprotein receptor-negative mice expressing human apolipoprotein B-100 develop complex atherosclerotic lesions on a chow diet: no accentuation by apolipoprotein(a). Proc Natl Acad Sci USA, 1998; 95: 4544-4549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92). González-Navarro H, Nong Z, Amar MJ, Shamburek RD, Najib-Fruchart J, Paigen BJ, Brewer HB, Jr, Santamarina-Fojo S: The ligand-binding function of hepatic lipase modulates the development of atherosclerosis in transgenic mice. J Biol Chem, 2004; 279: 45312-45321 [DOI] [PubMed] [Google Scholar]
- 93). Agellon LB, Walsh A, Hayek T, Moulin P, Jiang XC, Shelanski SA, Breslow JL, Tall AR: Reduced high density lipoprotein cholesterol in human cholesteryl ester transfer protein transgenic mice. J Biol Chem, 1991; 266: 10796-10801 [PubMed] [Google Scholar]
- 94). Kimura N, Kikumori A, Kawase D, Okano M, Fukamachi K, Ishida T, Nakajima K, Shiomi M: Species differences in lipoprotein lipase and hepatic lipase activities — Comparative studies of animal models of lifestyle-related diseases —. Exp Anim, 2019; 68: 267-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95). Sharyo S, Yokota-Ikeda N, Mori M, Kumagai K, Uchida K, Ito K, Burne-Taney MJ, Rabb H, Ikeda M: Pravastatin improves renal ischemia-reperfusion injury by inhibiting the mevalonate pathway. Kidney Int, 2008; 74: 577-584 [DOI] [PubMed] [Google Scholar]
- 96). Fujioka T, Tsujita Y, Shimotsu H: Induction of fatty acid synthesis by pravastatin sodium in rat liver and primary hepatocytes. Eur J Pharmacol, 1997; 328: 235-239 [DOI] [PubMed] [Google Scholar]
- 97). Essalmani R, Weider E, Marcinkiewicz J, Chamberland A, Susan-Resiga D, Roubtsova A, Seidah NG, Prat A: A single domain antibody against the Cys- and His-rich domain of PCSK9 and evolocumab exhibit different inhibition mechanisms in humanized PCSK9 mice. Biol Chem, 2018; 399: 1363-1374 [DOI] [PubMed] [Google Scholar]
- 98). Takahashi S, Ito T, Zenimaru Y, Suzuki J, Miyamori I, Takahashi M, Takahashi M, Ishida T, Ishida T, Hirata K, Yamamoto TT, Iwasaki T, Hattori H, Shiomi M: Species differences of macrophage very low-density-lipoprotein (VLDL) receptor protein expression. Biochem Biophys Res Commun, 2011; 407: 656-662 [DOI] [PubMed] [Google Scholar]
- 99). Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman D: Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol, 2010; 8: e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]


