Abstract
Background
Abivertinib is a novel oral, third generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) that overcomes T790M‐induced resistance in non‐small cell lung cancer (NSCLC) patients. Here, we report the results of a complete and detailed clinical data of patients treated with abivertinib at our hospital in a phase I dose escalation/expansion study of abivertinib.
Methods
NSCLC patients with the EGFR T790M mutation were orally administered abivertinib (150–300 mg) twice daily for cycles of 28 continuous days and tumor response was assessed. Further data regarding subsequent treatment protocols and survival were collected.
Results
A total of 28 NSCLC patients were included. Of the 24 assessable patients, 12 (50%) achieved a partial response (PR), and six (25%) achieved stable disease (SD). Median progression‐free survival (PFS) was 5.9 months (95% confidence interval (CI): 3.259–8.541) and median overall survival (OS) was 17.9 months (95% CI: 11.36–24.5). For salvage therapy in 15 (53.6%) patients after abivertinib, the median PFS following osimertinib treatment was 12 months. The median total treatment duration for the two third‐generation EGFR TKIs was 15.9 months (95% CI: 12.5–19.3). The most frequent abivertinib‐associated adverse effects were elevated hepatic transaminases (10/28, 35.7%) and diarrhea (10/28, 35.7%).
Conclusions
Abivertinib is a unique novel third‐generation EGFR TKI with good tolerance and efficacy in EGFR T790M(+) NSCLC patients. For patients with progressive disease after treatment with abivertinib, osimertinib could be an option for subsequent therapy but further studies are required.
Key points
Abivertinib is a novel third‐generation EGFR TKI targeting the EGFR T790M mutation
Abivertinib is well tolerated and efficacious in T790M‐positive patients
Abivertinib has a unique structure, efficacy, and resistance mechanism compared with osimertinib
Osimertinib treatment after AC0010 showed a good response
Keywords: Abivertinib, osimertinib, sequential treatment, T790M mutation, third‐generation EGFR TKI
Introduction
In the last two decades, due to the wide application of target therapy, prognosis for advanced‐stage non‐small cell lung cancer (NSCLC) has greatly improved.1 Epidermal growth factor receptor (EGFR) gene mutations occur in approximately 50% of Asian non‐small cell lung cancer (NSCLC) patients, particularly in never‐smokers and patients with adenocarcinoma.2 First‐line treatment with reversible first‐generation EGFR tyrosine kinase inhibitors (TKIs), such as gefitinib, erlotinib, and icotinib, and second‐generation EGFR TKIs (afatinib) have been shown to improve progression‐free survival (PFS) in NSCLC patients with EGFR‐sensitive mutations compared with chemotherapy.3, 4, 5, 6, 7, 8 However, despite the good initial responses of these first‐ and second‐generation EGFR TKIs, most patients develop acquired resistance. Moreover, there has been no evidence that patients benefit from the sequential use of different first‐generation EGFR TKIs.
The acquired EGFR T790M mutation accounts for 55%–70% of cases of resistance to first‐generation EGFR TKIs.9 However, third‐generation EGFR TKIs designed to specifically and selectively bind to and inhibit EGFR T790M are now available. Osimertinib is the first and only globally approved third‐generation EGFR TKI.10 It demonstrates good efficacy in NSCLC patients harboring acquired T790M mutations, although acquired resistance to osimertinib is also inevitable.
Abivertinib is a novel oral, potent, irreversible EGFR TKI that selectively targets EGFR mutations and overcomes T790M‐induced resistance in NSCLC patients.11, 12 Ma et al. reported the initial safety and efficacy of abivertinib in a single center phase I trial as well as its potential resistance mechanism from the results of plasma cell‐free DNA analysis, supporting its continued development.12 However, the objective response rate (ORR) was lower than that for osimertinib.13 The special resistance mechanism spectrum also indicated the difference between abivertinib and osimertinib.12, 13, 14 Moreover, little is known about sequential treatment with abivertinib and osimertinib.
To further investigate the therapeutic characteristics and significance of abivertinib, we reported the results of a complete and detailed clinical data analysis of patients who were enrolled at Peking Union Medical College Hospital in a phase I dose escalation/expansion study of abivertinib.
Methods
The study protocol of the phase 1 trial of abivertinib was approved by the Institutional Review Board (IRB) of Peking Union Medical College Hospital. All enrolled patients provided their written informed consent. The study adhered to the principles of the Declaration of Helsinki and the Good Clinical Practice guidelines. Specific written informed consent for the retrospective analysis about their subsequent therapy after abivertinib was waived by the IRB. No financial compensation was provided to patients.
Patient enrollment
Adult patients with a histologically or cytologically confirmed diagnosis of NSCLC, who had locally advanced or metastatic or relapsed NSCLC with a known EGFR TKI‐sensitizing mutation, had progressed from prior treatment with a first‐generation EGFR TKI (gefitinib, erlotinib, or icotinib), and had a central laboratory‐affirmed T790M mutation were enrolled to receive oral abivertinib in an expansion cohort in a phase I trial.
Tissue biopsies were required from patients progressing from prior EGFR TKI therapy. These tissue specimens were tested for EGFR T790M status in the Department of Pathology at Peking Union Medical College Hospital, using the amplification refractory mutation‐Scorpion system (Qiagen) and quantitative fluorescent PCR. Patients with a primary EGFR T790M mutation who had not been previously treated with an EGFR TKI were also eligible.
Abivertinib treatment and response assessment
Abivertinib was orally administered to patients in doses escalating from 150 mg to 300 mg twice daily for cycles of 28 continuous days until disease progression or unendurable toxicity.
Response was assessed on day 29 and then every eight weeks. The objective tumor response was assessed according to RECIST 1.1: a complete response (CR) was defined as the disappearance of all lesions; a PR was defined as a ≥30% decrease in the sum of the longest target lesion diameter, taking as reference the longest baseline diameter and/or the persistence of one or more nontarget lesions; Progressive disease (PD) was defined as a ≥20% increase in the sum of the longest diameter, taking as reference the smallest sum of the longest diameter recorded after treatment or the appearance of one or more new lesions, or the unequivocal progression of existing nontarget lesions; and SD was defined as the absence of significant shrinkage or enlargement qualifying as CR, PR, or PD, taking as reference the smallest sum of the longest diameter recorded after treatment. The PR or CR should be identified eight weeks (two cycles) from the first PR or CR assessment.
Subsequent treatment after progression on abivertinib
Further data about subsequent treatment and survival were collected prospectively after progression following abivertinib treatment, including the duration of subsequent treatment and overall survival. For patients subsequently treated with osimertinib, additional details were collected to investigate the benefit from osimertinib.
Statistical analysis
Descriptive analysis was performed for patient demographics. All time‐to‐event variables were estimated using the Kaplan‐Meier method. PFS was defined from the start of abivertinib treatment to disease progression or patient's death, whichever occurred first. OS was defined from the start of abivertinib treatment to the patient's death. Treatment duration of the third‐generation EGFR TKIs was defined as the treatment duration with abivertinib plus the treatment duration with osimertinib.
Data was updated on 1 January 2019 and statistical analyses performed using IBM SPSS Statistics for Windows (Version 19.0; SPSS Inc., Chicago, IL, USA).
Results
Patients' characteristics
Between August 2015 and August 2017, 28 patients were treated with abivertinib in the phase I dose‐escalation and expansion study at our hospital. Among them, 15 patients accepted subsequent antitumor therapy and nine patients were treated with osimertinib (Fig 1).
Figure 1.
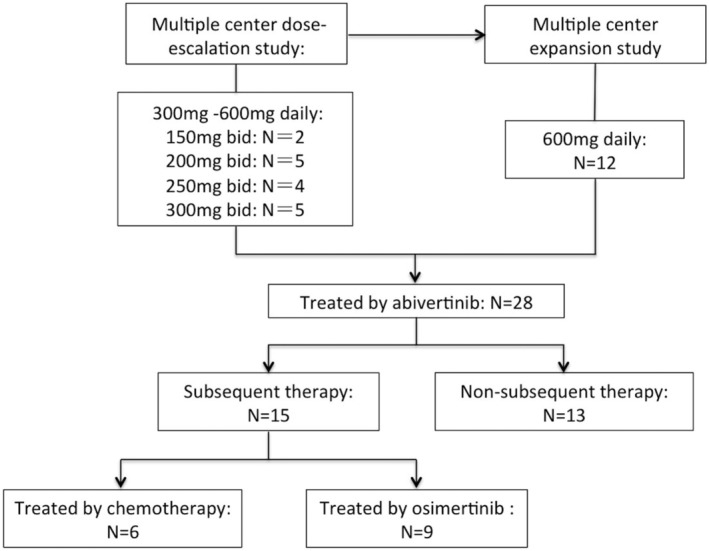
Study profile. A total of 28 patients were treated with abivertinib at Peking Union Medical College Hospital. After progression on abivertinib treatment, 15 patients accepted subsequent antitumor therapy and nine were treated with osimertinib.
General characteristics of the 28 patients are shown in Table 1. The median age was 58 years (range, 31–75 years); 14 patients (50.0%) were females, and eight (28.6%) were smokers. ECOG performance scores at baseline were 0–1 for all patients. A total of 14 (50%) patients had asymptomatic central nervous system (CNS) metastasis at baseline.
Table 1.
Patients' characteristics
| Characteristics | No. | Percent (%) |
|---|---|---|
| Age, years | ||
| Median | 58 | |
| Range | 31–75 | |
| Sex | ||
| Female | 14 | 50.0 |
| Male | 14 | 50.0 |
| Smoking | ||
| Yes | 8 | 28.6 |
| No | 20 | 71.4 |
| Initial EGFR mutation | ||
| 19del | 16 | 57.1 |
| L858 | 10 | 35.7 |
| 21L861Q/18G719X | 1 | 3.6 |
| 19del/T790M | 1 | 3.6 |
| Prior EGFR TKI | ||
| Gefitinib | 11 | 39.3 |
| Erlotinib | 2 | 7.1 |
| Icotinib | 14 | 50.0 |
| None† | 1 | 3.6 |
| Line of first generation TKIs | ||
| First | 23 | 85.2 |
| Second | 4 | 14.8 |
| Dose of abivertinib | ||
| 150 mg bid | 2 | 12.5 |
| 200 mg bid | 5 | 31.3 |
| 250 mg bid | 4 | 25.0 |
| 300 mg bid | 17 | 31.5 |
| Salvage therapy after abivertinib | ||
| Yes | 15 | 53.6 |
| No | 12 | 42.9 |
| Unknown | 1 | 3.6 |
The patient had de novo T790M mutation.
All patients had initial EGFR mutations: 16 patients had EGFR exon 19 deletions, 10 had exon 21 L858R mutations, one had concurrent exon 21 L861Q and exon 18 G719X mutations, and one had a de novo T790M mutation plus exon 19 deletion. A total of 27 patients had an acquired T790M mutation, and all had progressed from prior first‐generation EGFR TKIs (11 patients were treated with gefitinib, 14 with icotinib, and two with erlotinib; most were used as first‐line therapy). The only patient that had no prior therapy was the case with a de novo T790M mutation.
Tumor response to treatment with abivertinib and survival
Two patients receiving 600 mg of abivertinib daily withdrew because of severe adverse events (SAEs). Two patients chose to withdraw for personal reasons after two cycles of treatment; lesion shrinkages of more than 30% were evident in both patients according to their first assessment. The investigator's assessment of the remaining 24 patients showed that 12 (50%) achieved a PR, and six (25%) achieved stable disease (SD) (Fig 2). The ORR was 50% and the disease control rate was 75%.
Figure 2.
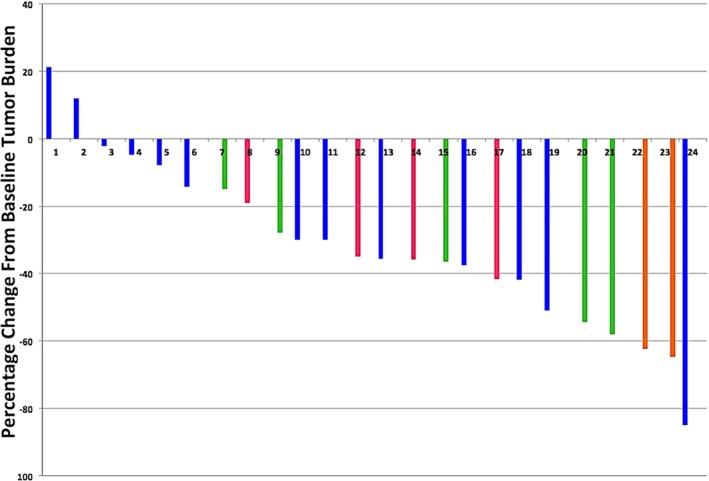
Waterfall plots for best percentage change in target lesion size in 28 patients. ( ) 300 mg bid, (
) 300 mg bid, ( ) 250 mg bid, (
) 250 mg bid, ( ) 200 mg bid and (
) 200 mg bid and ( ) 150 mg bid.
) 150 mg bid.
Until 1 January 2019, the mean follow‐up period was 462 days (range, 134–630 days). All 28 patients had progressed from abivertinib therapy, and 23 patients had died. The median PFS was 5.9 months (95% CI: 3.259–8.541), and the longest PFS was 18.9 months. The median OS was 17.9 months (95% CI: 11.36–24.5).
Adverse effects of treatment with abivertinib
All abivertinib‐associated adverse effects (AEs) in this study are shown in Table 2. The most frequent AEs were elevated hepatic transaminases (10/28, 35.7%) and diarrhea (10/28, 35.7%). Most AEs were mild (grade 1–2) and reversible. There were only four reversible grade 3 AEs (two rash, one diarrhea, and one interstitial lung disease [ILD]). Mild hematologic toxicities (neutropenia and thrombocytopenia, grade 1) were also found, and all were reversible. A total of 10 patients had skin toxicities, of whom two were grade 3. Considering the correlation between the dosage and incidence rate or severity of AEs, it seemed that most of the grade 3 AEs (one rash, one diarrhea, and one ILD) occurred at a dose of 600 mg daily. Two patients withdrew because of severe AEs (one rash and one ILD). Diarrhea was rare and mild at lower dosages (one case) but seemed more common or severe at higher dosages (nine cases at 600 mg daily). No dose reduction occurred.
Table 2.
Adverse events of every dosage (n = 28)
| Dose | 150 mg (n = 2) | 200 mg (n = 5) | 250 mg (n = 4) | 300 mg (n = 17) | Total N (%) |
|---|---|---|---|---|---|
| Rash | 0 | 0 | 2† | 3† | 5 (17.9%) |
| Pruritus | 1 | 0 | 0 | 2 | 3 (10.7%) |
| Skin chapped | 0 | 1 | 0 | 1 | 2 (7.1%) |
| Liver function abnormal | 2 | 2 | 3 | 3 | 10 (35.7%) |
| Nausea/vomiting | 1 | 0 | 1 | 1 | 3 (10.7%) |
| Abdominal distention | 0 | 1 | 0 | 2 | 3 (10.7%) |
| Loss of appetite | 0 | 1 | 0 | 0 | 1 (3.6%) |
| Constipation | 0 | 0 | 0 | 1 | 1 (3.6%) |
| Diarrhea | 0 | 1 | 0 | 9† | 10 (35.7%) |
| Neutropenia | 0 | 1 | 1 | 2 | 4 (14.3%) |
| Thrombocytopenia | 0 | 1 | 1 | 0 | 2 (7.1%) |
| Interstitial lung disease | 0 | 0 | 0 | 1† | 1 (3.6%) |
One of the adverse effect was grade 3 in each group, respectively.
Analysis of salvage therapy after progression with abivertinib treatment
Salvage therapy was administered to 15 (53.6%) patients after abivertinib ceased, while the remaining 13 (46.6%) patients failed to receive further antitumor treatment (three patients died of pulmonary infection, pulmonary embolism, and electrolyte disturbance, five patients were too ill to continue, and there were no suitable treatment options for four of the patients; osimertinib was not available in China at that time). Kaplan‐Meier analysis showed the median OS of the salvage therapy group was significantly longer than the best supportive care group (10.2 months [95% CI 5.8–14.6] vs. 17.9 months [95% CI 11.3–24.5]; P < 0.001).
Among the salvage therapy group, nine patients accepted osimertinib and the other six patients accepted chemotherapy.
The characteristics of the nine patients who accepted osimertinib are shown in Table 3. The median age was 62 years (range, 31–72 years); four patients (44.4%) were females. For prior treatment with abivertinib, all patients accepted abivertinib as their second‐line therapy, and all received a dose of 300 mg twice daily. Six patients stopped abivertinib because of progression, while two stopped because of SAEs (one ILD and one rash), and one stopped because of personal reasons. The median PFS following abivertinib treatment in six assessable patients was 2.9 months (range, 1.2–6.9 months). Three patients developed CNS metastasis after abivertinib treatment, but only one patient developed isolated CNS metastasis. One patient with symptomatic CNS metastasis accepted whole skull radiotherapy concurrently. No osimertinib‐related SAEs developed, and no relapse of ILD or severe rash occurred. Among them, six patients (66.7%) achieved a PR (Fig 3). The median PFS following osimertinib treatment was 12 months (four patients had not progressed at the time of publication). The median total treatment duration for the two third‐generation EGFR TKIs was 15.9 months (95% CI: 12.5–19.3) (Fig 4).
Table 3.
Clinical characteristics of the nine patients who accepted osimertinib after abivertinib
| Abivertinib | Osimertinib | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Age/Sex | Dose | Response | PFS (months) | Reasons for withdrawal | Site of progression | Response | Progression (Yes/No) | PFS (months) | OS (months) |
| 1 | 68M | 300 mg bid | SD‡ | 2.8 | ILD | — | PR | Yes | 12.0 | 18.9 |
| 2 | 72F | 300 mg bid | NA | 0.8 | Skin rash | — | PR | No | 15.0 | 29.8† |
| 3 | 63M | 300 mg bid | PR | 4.3 | Private reason | — | PR | Yes | 9.0 | 19.4 |
| 4 | 56M | 300 mg bid | PR | 6.9 | Progression | CNS, Lung§ | PR | Yes | 9.0 | 26.6 |
| 5 | 61F | 300 mg bid | PR | 4.0 | Progression | CNS | SD | No | 17.0 | 28.2† |
| 6 | 71F | 300 mg bid | PD | 2.9 | Progression | CNS, Lung, Liver | PD | Yes | 1.0 | 4.9 |
| 7 | 39F | 300 mg bid | PD | 1.2 | Progression | Lung | PR | No | 10.0 | 19.2† |
| 8 | 62M | 300 mg bid | PD | 2.8 | Progression | Lung | SD | Yes | 5.0 | 15.7 |
| 9 | 31M | 300 mg bid | PR | 5.7 | Progression | Lung | PR | No | 12.0 | 17.8† |
Patients who were alive at end date.
Not confirmed.
Patient accepted brain radiotherapy after progression occurred in the CNS.
Figure 3.
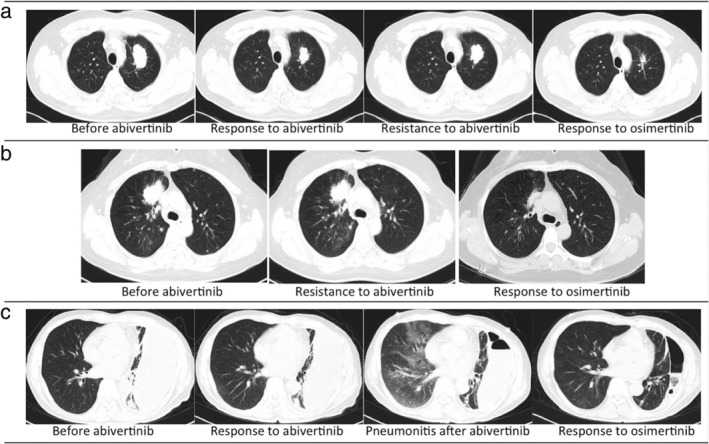
Changes in chest CT findings in some patients who accepted osimertinib after abivertinib treatment. (a) Patient No.9 achieved a PR from abivertinib treatment, with PFS of 170 days. Subsequent treatment with osimertinib achieved another PR, and PFS following osimertinib treatment was as long as one year (his disease was still under control). (b) Patient No.7 progressed after one month of abivertinib treatment; the target lesion in her lung remained stable while the nontarget lesion increased, then a PR was achieved after osimertinib treatment and PFS was longer than 10 months. (c) Patient No.1 developed interstitial lung disease after abivertinib treatment, with a response of SD following abivertinib; after the switch to osimertinib, a PR was achieved without the recurrence of ILD, and PFS following osimertinib treatment was 12 months.
Figure 4.
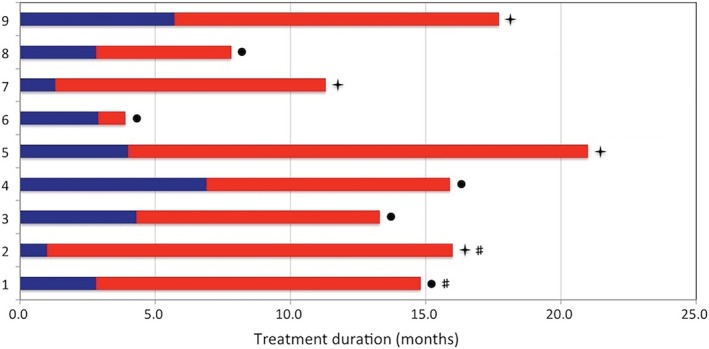
Treatment durations with abivertinib and sequential osimertinib in nine patients. ( ) PFS with abivertinib, (
) PFS with abivertinib, ( ) PFS with osimertinib, (
) PFS with osimertinib, ( ) disease progression, (
) disease progression, ( ) treatment ongoing and (
) treatment ongoing and ( ) switch because of adverse effect.
) switch because of adverse effect.
Discussion
The emergence of the gatekeeper EGFR T790M mutation, which prevents the binding of first‐generation EGFR TKIs to EGFR, has been demonstrated to be responsible for more than 50% of resistance to first‐ and second‐generation EGFR TKIs in NSCLC patients with EGFR‐sensitive mutations.15, 16, 17, 18, 19 The successful development of a third‐generation inhibitor that selectively targets the T790M mutation provides a good option for patients harboring the T790M mutation.20 Osimertinib is the only third‐generation EGFR TKI approved globally,10 and there are now several novel third‐generation EGFR TKIs in clinical development.20
Abivertinib is a novel, potential, irreversible EGFR TKI that is selective for the T790M mutation.11 The results of a total phase I trial of abivertinib were recently reported at the 56th ASCO meeting.21 The data of patients treated with abivertinib at Peking Union Medical College Hospital showed similar efficacy and safety. Without taking the different doses into account, abivertinib achieved an overall response rate of 50% and a disease control rate of 75%, which is superior to platinum‐based chemotherapy and comparable with other third‐generation EGFR TKIs such as osimertinib.20 The median PFS was 5.9 months (95% CI: 3.259–8.541), and the median OS was 17.9 months (95% CI: 11.36–24.5). These results indicated the efficacy of treatment with abivertinib, while PFS was a little shorter than that for osimertinib.13
The most common AEs were mild and included reversible elevated hepatic transaminases and diarrhea. Four severe AEs occurred, all of which were grade 3, and all patients recovered after the correct treatment. It is notable that there was a grade 3 ILD, and after complete recovery following treatment with prednisone, the subsequent therapy with osimertinib was successful and uneventful. Mild (grade 1) hematological toxicity was observed in several patients with no need for administration of a granulocyte‐colony stimulating factor. Taking into consideration the different dosages, diarrhea seemed to be more common at higher dosages (600 mg daily) than lower dosages, but most cases were mild and intermittent. No dose‐limiting toxicities occurred.
As for the sequential treatment of the patients who progressed after abivertinib treatment, it is interesting to note that the nine patients who accepted osimertinib as salvage therapy showed efficacy. The median PFS was as long as 12.0 months, with four patients maintained on osimertinib treatment to date. In two patients who experienced grade 3 ILD and skin rash because of abivertinib, no similar SAEs recurred during osimertinib therapy. The median total treatment duration with abivertinib and osimertinib was as long as 15.9 months, while the median response duration with osimertinib treatment alone was 12.3 months in NSCLC patients with T790M mutations according to a long‐term follow‐up of a pooled analysis of two phase II studies.22
To our knowledge, there is no evidence that the sequential use of different first generation TKIs (gefitinib, erlotinib, icotinib) could bring more benefits to patients, because all first‐generation TKIs target the same EGFR‐sensitive mutation. With regard to the effectiveness of osimertinib as a subsequent therapy after abivertinib, despite both being third‐generation EGFR TKIs, we supposed that it may be due to the unique structure of abivertinib, which differs from osimertinib.11
Compared with the structures of other reported third‐generation tyrosine kinase EGFR inhibitors (WZ4002, rociletinib, and osimertinib), abivertinib has a distinct chemical structure that contains a pyrrolopyrimidine ring system as its core, whereas all other third‐generation EGFR inhibitors, such as WZ4002, rociletinib, and osimertinib, have a pyrimidine core structure.11 Differences in this structure may lead to variation in the inhibition of the T790M mutation. Our results also indicated different toxicity spectrums between abivertinib and osimertinib, as the regimen switch was successful in two patients who developed SAEs from treatment with abivertinib. Regarding the potential resistance mechanism to abivertinib, there were also obvious differences between these two third‐generation EGFR TKIs according to an earlier small sample study.12 The cell‐free DNA sequencing results from 16 patients who developed resistance to abivertinib revealed that they harbored BRAF V600E mutations, ROS1 fusions, MNNG HOS transforming gene (MET), and erb‐b2 receptor tyrosine kinase 2 gene (ERBB2) amplification, but EGFR C797S mutations were not detected, which is a well‐known cause of resistance to osimertinib.23 Recently, Zhang et al. also reported the resistance mechanisms to abivertinib according to next‐generation sequencing (NGS)‐based genomic profiling of the plasma samples of 27 patients who developed abivertinib progression from the phase I dose‐escalation/expansion study. Their findings also reveal a heterogenous pattern of resistance mechanisms to abivertinib that is distinct from that previously reported with osimertinib, and EGFR amplification was the most common resistance mechanism in their cohort.14
We previously reported the low penetration rate of abivertinib through the blood‐brain barrier,24 which could cause poor efficacy in CNS metastasis, resulting in a short PFS, while osimertinib was reported to better control CNS metastasis.25 Therefore, in cases of isolated CNS metastasis, patients could still benefit from osimertinib. Among our nine patients, three developed CNS metastasis following abivertinib treatment, but only one developed isolated CNS metastasis, and the other two patients progressed with both intra‐ and extracranial disease concurrently, which indicated “real progression” on abivertinib; a PR was still achieved with osimertinib treatment in one patient. Furthermore, for the three patients without CNS metastasis after treatment with abivertinib, we still observed a benefit from subsequent osimertinib treatment. Thus, the poor penetration of abivertinib through the blood‐brain barrier could not explain all the cases since only some patients developed brain metastases after abivertinib treatment.
Thus, the clinically significant benefit from subsequent treatment with osimertinib among our patients implies that abivertinib progression may be due to incomplete target inhibition, and the efficacy of abivertinib seemed weaker than that of osimertinib. The report by Zhang et al.14 showed that EGFR T790M loss (15%) with abivertinib resistance was much less frequent than that reported in osimertinib resistance cohorts (42%–68%),26 which verified the incomplete inhibition of abivertinib compared with osimertinib and also explained our findings regarding the response to osimertinib after abivertinib progression.
The main limitation of our study was the small sample size. Consequently, the results of this study may not be thoroughly representative and further studies with a larger sample size are needed. Although we observed the phenomenon that patients could benefit from subsequent osimertinib after progression on abivertinib treatment, suggesting the possibility of a therapeutic strategy, further well‐designed clinical studies are required. Furthermore, osimertinib was recently approved and is being broadly adapted as the first‐line therapy for all advanced EGFR mutant non‐small cell lung cancers.27 When osimertinib is used as the first‐line therapy, these patients will never develop T790M mutation. However, China has a large number of adenocarcinoma patients with EGFR positive mutations, and there will still be many patients with EGFR positive mutations who choose to start treatment with first‐ or second‐generation TKIs. As a third‐generation TKI with Chinese proprietary property, abivertinib still has important development prospects.
In conclusion, abivertinib is a unique novel EGFR TKI. It is well tolerated and efficacious in EGFR T790M(+) NSCLC patients. For patients who progress after treatment with abivertinib, osimertinib could be a subsequent therapy option but further studies are required.
Disclosure
The authors declare that they have no conflicts of interest regarding this work.
Acknowledgments
The authors are grateful to the sponsor Hangzhou ACEA Pharmaceutical Research Co., Ltd. for enabling us to undertake this work. We thank the patients who participated in this study. We also thank H Nikki March, PhD, from Liwen Bianji, Edanz Editing China (http://www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
References
- 1. Asselain B, Barrière JR, Clarot C et al Metastatic NSCLC: Clinical, molecular, and therapeutic factors associated with long‐term survival. Respir Med Res 2019; 76: 38–44. 10.1016/j.resmer.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 2. Han B, Tjulandin S, Hagiwara K et al Determining the prevalence of EGFR mutations in Asian and Russian patients (pts) with advanced non‐small‐cell lung cancer (ANSCLC) of adenocarcinoma (ADC) and non‐ADC histology: IGNITE study. Ann Oncol 2015; 26 (Suppl. 1): i29–30. [DOI] [PubMed] [Google Scholar]
- 3. Fukuoka M, Wu YL, Thongprasert S et al Biomarker analyses and final overall survival results from a phase III, randomized, open‐label, first‐line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non‐small‐cell lung cancer in Asia (IPASS). J Clin Oncol 2011; 29 (21): 2866–74. [DOI] [PubMed] [Google Scholar]
- 4. Mok TS, Wu YL, Thongprasert S et al Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947–57. [DOI] [PubMed] [Google Scholar]
- 5. Zhou C, Wu YL, Chen G et al Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐ cell lung cancer (OPTIMAL, CTONG‐0802): A multicentre, open‐label, randomised, phase 3 study. Lancet Oncol 2011; 12 (8): 735–42. [DOI] [PubMed] [Google Scholar]
- 6. Shi Y, Zhang L, Liu X et al Icotinib versus gefitinib in previously treated advanced non‐small‐cell lung cancer (ICOGEN): A randomised, double‐ blind phase 3 non‐inferiority trial. Lancet Oncol 2013; 14 (10): 953–61. [DOI] [PubMed] [Google Scholar]
- 7. Shi YK, Wang L, Han BH e a. First‐line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation‐positive lung adenocarcinoma (CONVINCE): A phase 3, open‐label, randomized study. Ann Oncol 2017; 28 (10): 2443–50. [DOI] [PubMed] [Google Scholar]
- 8. Sequist LV, Yang JCH, Yamamoto N et al Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31 (27): 3327–34. [DOI] [PubMed] [Google Scholar]
- 9. Yu HA, Arcila ME, Rekhtman N et al Analysis of tumor specimens at the time of acquired resistance to EGFR‐TKI therapy in 155 patients with EGFR‐mutant lung cancers. Clin Cancer Res 2013; 19: 2240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Greig SL. Osimertinib: First global approval. Drugs 2016; 76 (2): 263–73. [DOI] [PubMed] [Google Scholar]
- 11. Xu X, Mao L, Xu W et al AC0010, an irreversible EGFR inhibitor selectively targeting mutated EGFR and overcoming T790M‐induced resistance in animal models and lung cancer patients. Mol Cancer Ther 2016; 15 (11): 2586–97. [DOI] [PubMed] [Google Scholar]
- 12. Ma Y, Zheng X, Zhao H et al First‐in‐human phase I study of AC0010, a mutant‐selective EGFR inhibitor in non‐small cell lung cancer: Safety, efficacy, and potential mechanism of resistance. J Thorac Oncol 2018; 13 (7): 968–77. 10.1016/j.jtho.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 13. Mok TS, Wu Y‐L, Ahn M‐J et al Osimertinib or platinum‐pemetrexed in EGFR T790M‐positive lung cancer. N Engl J Med 2017; 376 (7): 629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang YC, Chen ZH, Zhang XC et al Analysis of resistance mechanisms to abivertinib, a third‐generation EGFR tyrosine kinase inhibitor, in patients with EGFR T790M‐positive non‐small cell lung cancer from a phase I trial. EBioMedicine 2019; 43: 180–7. 10.1016/j.ebiom.2019.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yun CH, Mengwasser KE, Toms AV et al The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A 2008; 105: 2070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gazdar A. Activating and resistance mutations of EGFR in non‐small‐cell lung cancer: Role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene 2009; 28 (Suppl. 1): S24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Niederst MJ, Engelman JA. Bypass mechanisms of resistance to receptor tyrosine kinase inhibition in lung cancer. Sci Signal 2013; 6: re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kobayashi S, Boggon TJ, Dayaram T et al EGFR mutation and resistance of non–small‐cell lung cancer to gefitinib. N Engl J Med 2005; 352: 786–92. [DOI] [PubMed] [Google Scholar]
- 19. Pao W, Miller VA, Politi KA et al Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLOS Med 2005; 2: e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tan CS, Kumarakulasinghe NB, Huang YQ et al Third generation EGFR TKIs: Current data and future directions. Mol Cancer 2018; 17 (1): 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou Q, Wu L, Feng L et al Safety and efficacy of abivertinib (AC0010), a third‐generation EGFR tyrosine kinase inhibitor, in Chinese patients with EGFR‐T790M positive non‐small cell lung cancer (NCSLC). J Clin Oncol 2019; 37: (Suppl.): Abstract 9091. [Google Scholar]
- 22. Ahn MJ, Tsai CM, Shepherd FA et al Osimertinib in patients with T790M mutation‐positive, advanced non–small cell lung cancer: Long‐term follow‐up from a pooled analysis of 2 phase 2 studies. Cancer 2019; 125 (6): 892–901. 10.1002/cncr.31891. [DOI] [PubMed] [Google Scholar]
- 23. Thress KS, Paweletz CP, Felip E et al Acquired EGFR C797S mutation mediates resistance to AZD9291 in non small cell lung cancer harboring EGFR T790M. Nat Med 2015; 21: 560–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang H, Zhang L, Hu P et al Penetration of the blood‐brain barrier by avitinib and its control of intra/extra‐cranial disease in non‐small cell lung cancer harboring the T790M mutation. Lung Cancer 2018; 122: 1–6. 10.1016/j.lungcan.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 25. Tan CS, Cho BC, Soo RA. Treatment options for EGFR mutant NSCLC with CNS involvement‐can patients BLOOM with the use of next generation EGFR TKIs. Lung Cancer 2017; 108: 29–37. [DOI] [PubMed] [Google Scholar]
- 26. Zhou C, Hu M, Zhu X et al Resistance mechanisms of osimertinib in Chinese non‐small cell lung cancer patients: Analysis from AURA17 trial. J Thorac Oncol 2018; 13 (10S): S345. [Google Scholar]
- 27. Soria JC, Ohe Y, Vansteenkiste J et al Osimertinib in untreated EGFR‐mutated advanced non‐small‐cell lung cancer. N Engl J Med 2018; 378 (2): 113–25. 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]


