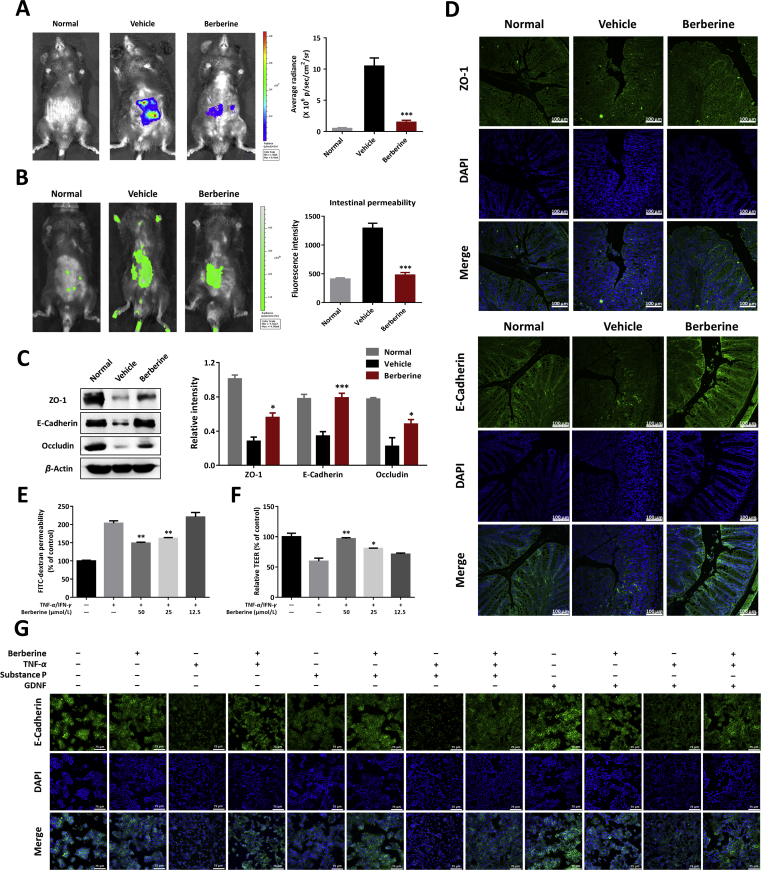
Figure 3.
Berberine maintained the intestinal barrier homeostasis in DSS-colitis mice and TNF-α and/or neuropeptides-cultured epithelial cells. (A) Left, typical in vivo imaging pictures with bioluminescent imaging by injecting L-012 sodium (25 mg/kg). Right, quantification of bioluminescent images by ROI determination. (B) Intestinal permeability measurement by oral administration of FITC-dextran (600 mg/kg). Fluorescent images (left) and serum fluorescence intensity (right) of FITC-dextran were determined. (C) Left, colonic homogenates were analyzed by Western blotting to detect the expression of tight junction protein (ZO-1, E-cadherin, and occludin). Right, quantitative analysis of tight junction protein expression. (D) Immunofluorescence staining with ZO-1 and E-cadherin of colonic sections (Scale = 100 μm). (E) and (F) Barrier function in vitro was assayed in TNF-α and IFN-γ-primed Caco-2 cells. FITC-dextran permeability (E) and trans-epithelial electrical resistance (TEER, F) were determined. (G) HT-29 cells were cultured with berberine, substance P, or GDNF in the absence or presence of TNF-α induction. E-cadherin expression by immunofluorescence staining was performed (Scale = 75 μm). (A)–(D), all data were presented as mean ± SEM; n = 8 mice per group. (E)–(G), all data were presented as mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001: (A)–(C), compared with the vehicle group; (E) and (F), compared with TNF-α/IFN-γ-stimulated cells.