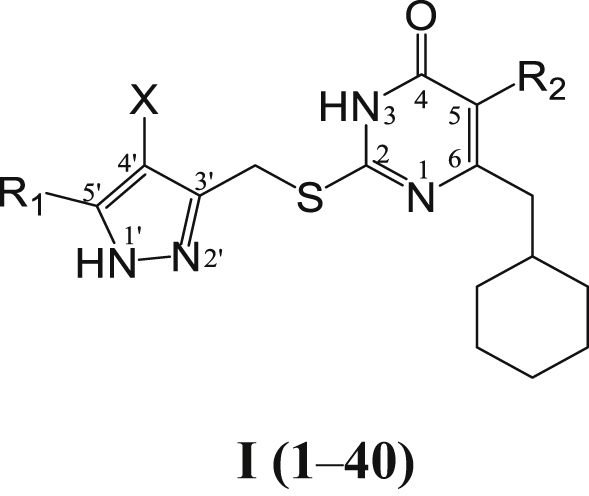
Table 1.
Activity against HIV-1IIIB strain, cytotoxicity and SI of the title compounds in C8166 cellsa.
| Compd. | R1 | R2 | X | EC50b (μmol/L) | CC50c (μmol/L) | SId |
|---|---|---|---|---|---|---|
| I-01 | Ph | Et | H | 0.0068 ± 0.0017 | 84.48 ± 0.91 | 12,424 |
| I-02 | 3′-Me-Ph | Et | H | 0.0739 ± 0.0079 | 82.76 ± 2.20 | 1120 |
| I-03 | 3′-Br-Ph | Et | H | 0.0512 ± 0.0073 | 76.26 ± 3.09 | 1489 |
| I-04 | 3′-Cl-Ph | Et | H | 0.0665 ± 0.0388 | 80.99 ± 0.38 | 1218 |
| I-05 | 3′-F-Ph | Et | H | 0.0348 ± 0.0206 | 71.99 ± 8.86 | 2069 |
| I-06 | 3′-CF3-Ph | Et | H | 0.3016 ± 0.2135 | 34.42 ± 3.50 | 114 |
| I-07 | 4′-Me-Ph | Et | H | 0.0523 ± 0.0385 | 91.42 ± 1.90 | 1748 |
| I-08 | 4′-Cl-Ph | Et | H | 0.0178 ± 0.0112 | 83.86 ± 0.45 | 4711 |
| I-09 | 4′-F-Ph | Et | H | 0.0200 ± 0.0018 | 74.93 ± 4.82 | 3747 |
| I-10 | 4′-MeO-Ph | Et | H | 0.0232 ± 0.0071 | >200 | >8621 |
| I-11 | 4′-OH-Ph | Et | H | 0.0038 ± 0.0011 | 96.78 ± 8.17 | 25,468 |
| I-12 | 4′-MeS-Ph | Et | H | 0.0118 ± 0.0088 | >200 | >16,949 |
| I-13 | 4′-(CH3)2CH-Ph | Et | H | 0.1469 ± 0.0552 | 31.12 ± 3.81 | 212 |
| I-14 | 3′,4′-diCl-Ph | Et | H | 0.3102 ± 0.2008 | 75.35 ± 8.48 | 243 |
| I-15 | 3′,4′-diF-Ph | Et | H | 0.1617 ± 0.0371 | 86.43 ± 3.10 | 535 |
| I-16 | 2′,4′-diMe-Ph | Et | H | 0.4759 ± 0.4129 | 87.39 ± 1.87 | 184 |
| I-17 | 2′,4′-diF-Ph | Et | H | 0.0418 ± 0.0031 | 59.71 ± 8.78 | 1428 |
| I-18 | 2′-N-pyridyl | Et | H | 0.0228 ± 0.0017 | 79.44 ± 5.18 | 3484 |
| I-19 | H | Et | H | 0.0334 ± 0.0056 | 75.01 ± 6.88 | 2246 |
| I-20 | Me | Et | H | 0.0277 ± 0.0015 | 45.16 ± 10.71 | 1630 |
| I-21 | Cyclopropyl | Et | H | 0.0245 ± 0.0027 | 47.19 ± 4.89 | 1926 |
| I-22 | C(CH3)3 | Et | H | 0.2965 ± 0.1034 | 67.93 ± 4.76 | 229 |
| I-23 | Ph | Et | Cl | 0.0990 ± 0.0019 | >200 | >2020 |
| I-24 | 3′-Me-Ph | Et | Cl | 0.0860 ± 0.0152 | 77.10 ± 13.61 | 897 |
| I-25 | 3′-Br-Ph | Et | Cl | 0.1015 ± 0.0025 | 58.06 ± 9.84 | 572 |
| I-26 | 3′-Cl-Ph | Et | Cl | 0.0570 ± 0.0033 | 66.08 ± 31.43 | 1159 |
| I-27 | 3′-F-Ph | Et | Cl | 0.0713 ± 0.0436 | >200 | >2805 |
| I-28 | 3′-CF3-Ph | Et | Cl | 0.1819 ± 0.0116 | 55.05 ± 3.82 | 303 |
| I-29 | 4′-Me-Ph | Et | Cl | 0.1380 ± 0.0469 | >200 | >1449 |
| I-30 | 4′-Cl-Ph | Et | Cl | 0.0893 ± 0.0629 | >200 | >2240 |
| I-31 | 4′-F-Ph | Et | Cl | 0.0181 ± 0.0017 | 64.92 ± 14.55 | 3587 |
| I-32 | 3′,4′-diCl-Ph | Et | Cl | 0.3495 ± 0.0197 | 190.20 ± 13.85 | 544 |
| I-33 | 3′,4′-diF-Ph | Et | Cl | 0.1512 ± 0.0960 | 115.43 ± 19.05 | 763 |
| I-34 | 4′-MeO-3′-Cl-Ph | Et | Cl | 0.1299 ± 0.0508 | 49.84 ± 8.21 | 384 |
| I-35 | Ph | CH3 | H | 0.1865 ± 0.0936 | 54.62 ± 2.88 | 293 |
| I-36 | 4′-MeO-Ph | CH3 | H | 0.1941 ± 0.0103 | 66.88 ± 0.86 | 345 |
| I-37 | 4′-F-Ph | CH3 | H | 0.3552 ± 0.1654 | 42.23 ± 1.75 | 119 |
| I-38 | Ph | H | H | 4.9825 ± 2.5257 | 79.06 ± 5.34 | 16 |
| I-39 | 4′-MeO-Ph | H | H | 3.9567 ± 2.0214 | 56.05 ± 0.89 | 14 |
| I-40 | 4′-F-Ph | H | H | 10.0751 ± 4.1284 | 61.62 ± 3.22 | 6 |
| DB02 | – | – | – | 0.0067 ± 0.0021 | >200 | >29,851 |
| ETR | – | – | – | 0.0014 ± 0.0034 | 27.48 ± 5.69 | 19,628 |
| NVP | – | – | – | 0.0402 ± 0.0257 | >200 | >4975 |
| AZT | – | – | – | 0.0089 ± 0.0002 | >200 | >22,472 |
–Not applicable.
All data represent as mean ± SD (n = 3).
Effective concentration required to protect C8166 cell against the cytopathogenicity of HIV by 50%.
Cytostatic concentration required to reduce C8166 cell proliferation by 50% tested by MTT method.
Selectivity index: ratio CC50/EC50, a higher SI means a more selective compound.
