-
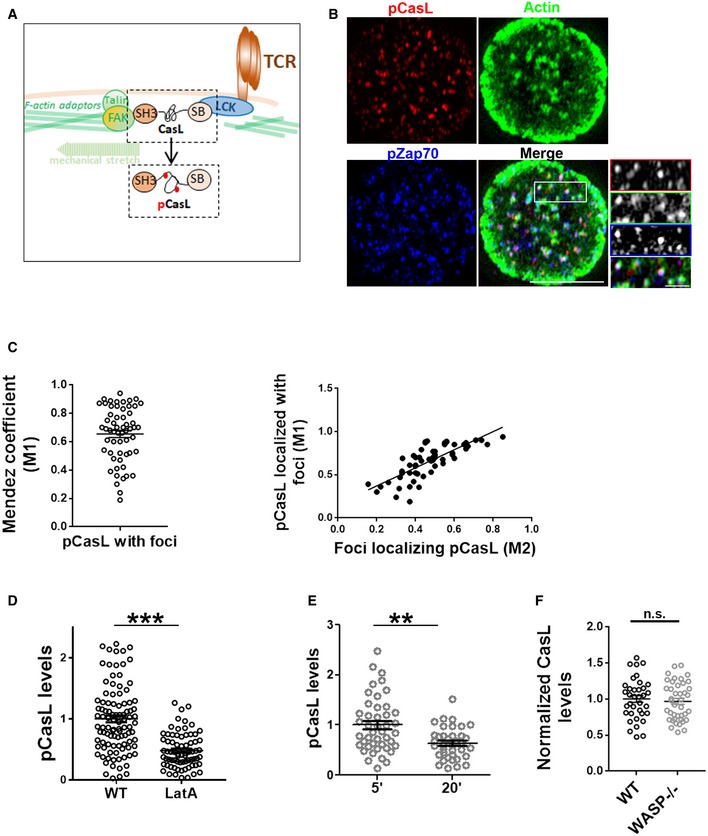
A
A schematic of mechanosensitive CasL phosphorylation in T cells. CasL is recruited to the signaling TCR clusters via LCK via its Src kinase binding domain (SB) and interacts with the F‐actin cytoskeleton and adhesion complex binding proteins such as FAK via its SH3 domain. Mechanical tension created due to actin polymerization and actomyosin contraction at the foci sites leads to conformational changes in CasL, exposing tyrosine motifs in its substrate domain. These tyrosine residues are phosphorylated by the local Src family kinases and could be immunolabelled to assess TCR‐proximal actin cytoskeletal tension.
-
B, C
Colocalization index of foci and pCasL shows a high degree of association (left plot in C), and correlation with foci intensity per cell (right plot in C). Scale bar, 5 μm.
-
D
pCasL levels are sensitive to broad actin cytoskeletal perturbation. T cells from WT mice were treated with latrunculin A (LatA), or left untreated, during incubation with substrate. Cells were subsequently fixed and processed for immunostaining and imaged using TIRF microscopy. ***P < 0.0001, measured using Mann–Whitney test.
-
E
T cells show reduced pCasL in their polarized synapses. **P = 0.001, measured using Mann–Whitney test.
-
F
Recruitment of CasL to the synapse is not reduced in WASP−/− T cells. P value, ns = 0.58, as measured using Mann–Whitney test. The points in (D–F) are the values obtained from individual cells normalized to mean of WT (D) or 5′ (E) values.