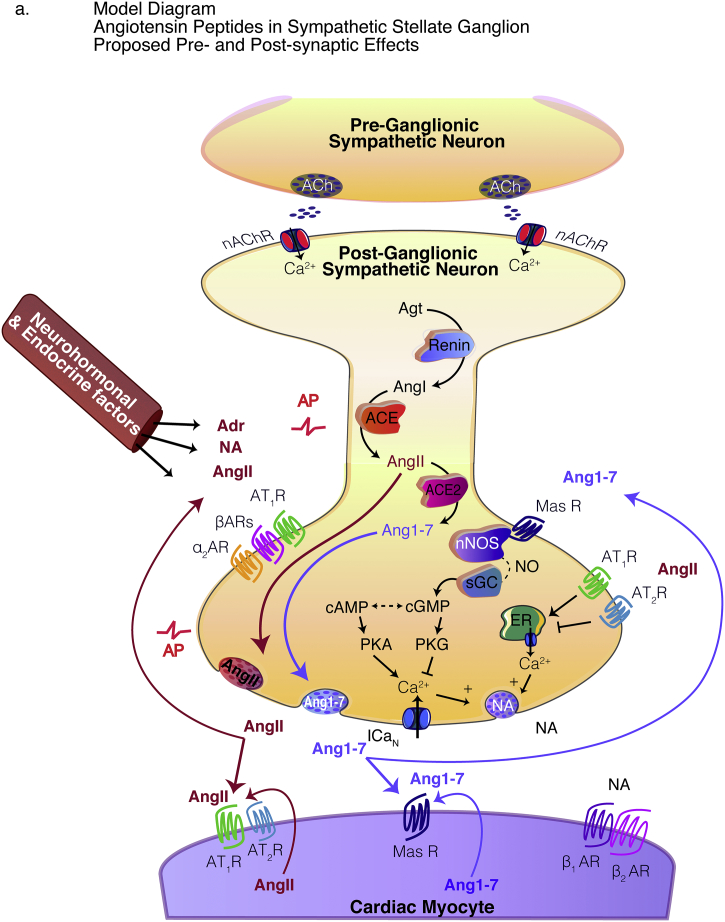
Fig. 5.
Model diagram depicts angiotensin synthesis and pre- and post-synaptic signaling pathways.
In sympathetic stellate neurons, the classical pathway for Angiotensin II (AngII) synthesis occurs by sequential enzymatic cleavage of Angiotensinogen (Agt) by renin and Angiotensin Converting Enzymes (ACE). AngII is hydrolyzed by ACE2 producing the bioactive metabolite of Angiotensin 1–7 (Ang1–7). We identified the presence of precursors and transcripts encoding these enzymes and depict here a proposed model for angiotensin synthesis (a). We also identified the presence of AngII and Ang1–7 receptors on sympathetic stellate ganglia of human and rat. AngII has been shown to elevate intracellular Ca2+ and enhance noradrenaline release via actions at AT1R [59,60]. Conversely Ang1–7-dependent activation of its cognate receptor, Mas R, has been shown to couple to NO in the brain and several other receptor sites [61]. In this study, we show that administration of both AngII and Ang1–7 elevate cGMP in the rat stellate ganglia. We and others have previously demonstrated the importance of NO-cGMP signaling in reducing [Ca2+]i [47,63] and end-organ transmission in peripheral sympathetic stellate nerves [47,53,64,65] although the effects of Ang1–7 may be biphasic [66]. Dotted lines indicate intermediates in these intracellular signaling pathway. Several effects of AngII and Ang1–7 on the myocardium have been established [22,[67], [68], [69], [70], [71]].